
Dear Advocates,
We are excited to welcome you to the fourth edition of the Friends Advocacy Newsletter. It has been a great year working with you to create and launch this resource and we want to hear your thoughts on what you have liked, what could be improved, and what you want to see more of. We hope you have a wonderful holiday season and look forward to continuing our work in 2024!
As we look forward to continuing the Friends Advocacy Newsletter in 2024, we encourage you to look through newsletters from the past year and provide feedback:
Here’s what you can read more about in this newsletter:
- What’s Happening at Friends?: Friends Advisory Advocates who contributed to our 2023 Annual Meeting topics share their perspectives.
- Important News in Cancer Research: Recent news, FDA activities, and policy developments highlighting topics related to Friends’ work and topics impacting cancer care and drug development.
- 2023 Year in Review: Timeline of 2023 Accomplishments and Advocate Impacts.
- In Case You Missed It: Recaps of recent events and links to new publications and content from Friends.
- Mark Your Calendars: Upcoming events, including public meetings hosted by Friends and other relevant stakeholders.
What's Happening at Friends in 2023?
In this section, we’re pleased to highlight the perspectives of four dedicated patient advocates who made invaluable contributions to our working groups and discussions for the Friends of Cancer Research 2023 Annual Meeting, which took place on November 14, 2023. As part of these multi-stakeholder working groups, they attended biweekly virtual meetings leading up to the event and helped to develop white papers detailing discussions and providing recommendations to address critical issues in the development of new oncology drugs. Through sharing the patient’s perspective, these advocates played a pivotal role in shaping recommendations that will contribute to progress in cancer clinical trials and care.
Incorporating Pragmatic Trial Elements into Oncology Drug Development
 Kelly Shanahan, MD, Living with metastatic breast cancer, METAvivor Board of Directors
Kelly Shanahan, MD, Living with metastatic breast cancer, METAvivor Board of Directors
I happily jumped on the opportunity to participate in the pragmatic trials working group because [patients] are the ultimate stakeholders. We are the people participating in trials, and we need more people of diverse backgrounds if trials are going to be applicable to the real world. Who better to provide guidance on pragmatic trials than the people you hope to enroll?
 Kristin R. McJunkins, M.Ed., Patient Advocate
Kristin R. McJunkins, M.Ed., Patient Advocate
In the last decade or so, there has been an increase in targeted therapy and precision medicine. As an advocate, participating in this working group has increased my understanding from the provider perspective of the complexities involved with designing trials in the best interest of the patient.
Access to clinical trials is challenging for many people, particularly if they don’t live near a vibrant academic medical center. Allowing more flexibility in who can help administer the trial will potentially increase the number of people who are able to participate and, ideally, increase access to care and positive treatment outcomes.
Early Phase Trials: Data Implementation and Interpretation for Dose-Finding
 Judy Medeiros Fitzgerald, Breast Cancer Advocate and Founder of Sisters4Prevention
Judy Medeiros Fitzgerald, Breast Cancer Advocate and Founder of Sisters4Prevention
The inclusion of consumer advocates in trial protocols and development is essential and necessary to represent the patient perspective. Often researchers do not have the opportunity to meet or work directly with patients. Consequently, they may not realize the impact of trial protocols and harms experienced on patients’ quality of life. This is the important mission of patient advocates, and we are grateful to have the opportunity to represent the patient voice.
Until recently, clinical trials only recommended and reported what is/was referred to as the “Maximum Tolerated Dose.” This MTD is/was the highest dose the patient could tolerate with minimal harms. Presently, the paradigm has shifted towards “Individualized Oncology” and “Dose Optimization.” This change has proven to be revolutionary and often life saving for patients. Sanctioning individualized lower doses based on the patient’s health or body weight, by no longer excluding patients with pre-existing conditions or prior chemotherapy and improving efforts for better reporting of “quality of life,” are all goals which are imperative to patients’ health. These should be regarded by researchers and oncologists as paramount in developing future trials and treatment protocols for patients.
Maximizing Use of Data from Academic-Led Studies for Regulatory Decision-Making
 Abigail M. Johnston, Esquire, MBC Patient Advocate
Abigail M. Johnston, Esquire, MBC Patient Advocate
The perspective of the end user, the patient, is often overlooked and having the ability to provide that perspective is so important to me. The more patients understand the inner workings of clinical trials, the more patients will feel more comfortable participating. We need more patients to bring the end user perspective from the beginning of clinical trials to the point where patients are enrolled.
To learn more about our 2023 Annual Meeting Topics, read the white papers and rewatch the meeting here.
Important News in Cancer Research
- In November, Dr. Monica Bertagnolli was confirmed as the 17th Director of the National Institutes of Health (NIH). Dr. Bertagnolli previously served as the Director of the National Cancer Institute (NCI). She is the first surgeon and second woman to lead the NIH. Among her key priorities as NIH Director is improving diversity in clinical trials to ensure they produce results that can benefit all patients. Read more.
- In November, Dr. Monica Bertagnolli was confirmed as the 17th Director of the National Institutes of Health (NIH). Dr. Bertagnolli previously served as the Director of the National Cancer Institute (NCI). She is the first surgeon and second woman to lead the NIH. Among her key priorities as NIH Director is improving diversity in clinical trials to ensure they produce results that can benefit all patients. Read more.
- In September, the FDA announced a proposed rule aimed at ensuring the safety and reliability of laboratory developed tests (LDTs). For more context and insights on what this rule means for patients, read here.
- The FDA’s Oncologic Drugs Advisory Committee (ODAC) met to discuss delayed confirmatory trials for two drugs that received accelerated approval (AA). The committee also discussed and identified opportunities to optimize the AA pathway with a focus on decreasing the amount of time it takes drugs to verify clinical benefit.
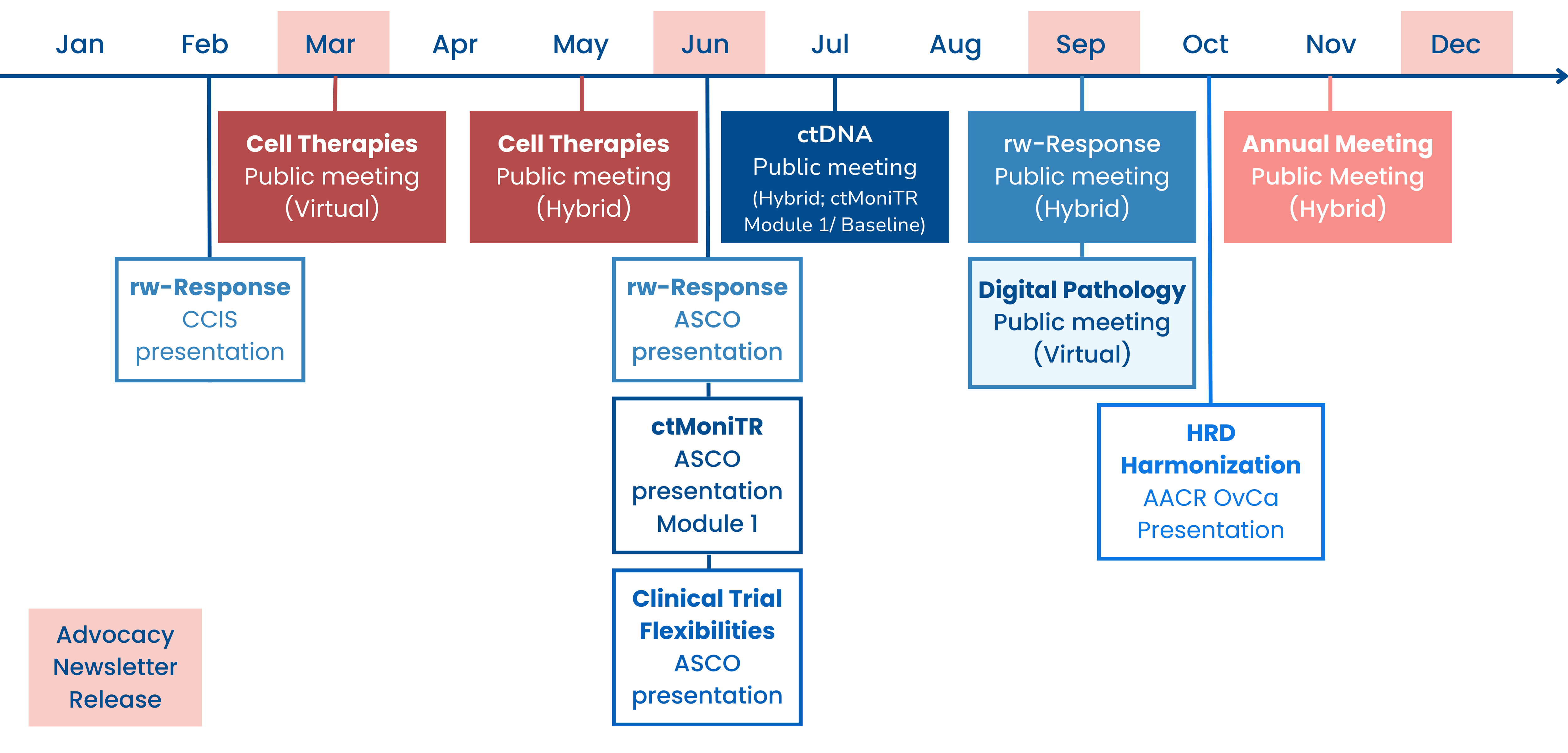
2023 Year in Review
In Case You Missed It (September 15th - December 15th)
- Friends published a new analysis in Clinical Cancer Research exploring how postmarketing requirements (PMR) are used for conducting further dose optimization of novel oncology drugs after they are approved. Read the publication here or explore more data on PMRs on Friends’ new dashboard here.
- Friends leads initiatives that advance the use of real-world data (RWD) and evidence (RWE). We created a new one-pager to provide a comprehensive overview of this work, read here. Learn more about our RWE portfolio here.
- As was discussed in depth in our last newsletter, on September 26th Friends hosted a virtual meeting to discuss opportunities for the use of digital pathology and computational pathology in oncology drug development. For details on what was discussed during the meeting read the white paper or read the meeting recap and rewatch the meeting. Learn more about our digital pathology work here.
- In October, Friends presented results from our ongoing HRD Harmonization Project at the AACR Special Conference: Ovarian Cancer. Learn more about our HRD Harmonization Project here.
Mark Your Calendars
If you’re interested in supporting an event hosted by Friends, please email advocacy@focr.org
January 31st – February 1st, 2024 – Current Developments in Digital Health Technology and Regulation Conference
February 1st – February 29th, 2024 – National Cancer Prevention Month
^ February 1st, 2024 – Friends Public Meeting, The Future of Diagnostic Tests: New Data & Modern Policy
^ February 15th, 2024 – Friends Public Meeting, Enhancing Diversity in Clinical Trials: Implementation of Diversity Plans
^ February 20th, 2024 – Advancing Real-World Data in Oncology Workshop
^ February 28th, 2024 – Enhancing Capacity for Primary Care Research in Cancer Survivorship: A Workshop for Action
^ March 5th, 2024 – Advancing the Use of Complex Innovative Designs in Clinical Trials: From Pilot to Practice
^ March 19 – 20th, 2024 – Enhancing Adoption of Innovative Clinical Trial Approaches
* = Reduced Registration for Advocates
^ = Free to Attend
† = Friends Staff Presenting
ProgressforPatients.org
ProgressforPatients.org is an online advocacy education program created by Friends to help patients, advocates, and caregivers acquire the necessary tools to effectively communicate with drug researchers, developers, and regulators. If you’re interested, we encourage you to take our free advocacy education course found here.

