ctMoniTR Project
ctDNA for Monitoring Treatment Response Project
As treatments continue to improve, clinical trials that use overall survival as an endpoint become longer, which may delay new safe and effective drugs from getting to patients. To overcome this, the Accelerated Approval pathway allows for approval based on an intermediate endpoint that is reasonably likely to predict response to treatment. Circulating tumor DNA (ctDNA) is fragments of DNA shed from cancer cells, found in the bloodstream, and collected using a blood draw. Change in ctDNA levels on treatment has the potential to be used as an intermediate endpoint, however, aggregate data analyses are necessary to ensure the field is confident with using ctDNA as an intermediate endpoint.
Several organizations have performed clinical trials exploring whether change in ctDNA levels on treatment associates with overall survival in cancer, however, these have been small studies which limit the analyses and conclusions that can be drawn. To leverage the data from multiple studies for robust evidence generation, it is critical that these data are aligned to ensure measurement of ctDNA is cohesive and consistent across groups.
Friends leads a multi-stakeholder research project, ctMoniTR, with pharmaceutical companies, diagnostic labs, government health officials, patient advocates, and academic researchers. We collect data from clinical trials that incorporate ctDNA as a tool to track how advanced disease responds to treatment. Our collaboration with the statisticians at Cancer Research And Biostatistics (CRAB) assesses whether ctDNA change reflects response to treatment across multiple clinical trials. By combining efforts and aggregating data across multiple trials, we will be able to generate the evidence necessary to characterize ctDNA as an indicator of response faster than if any single organization tried to do so alone.
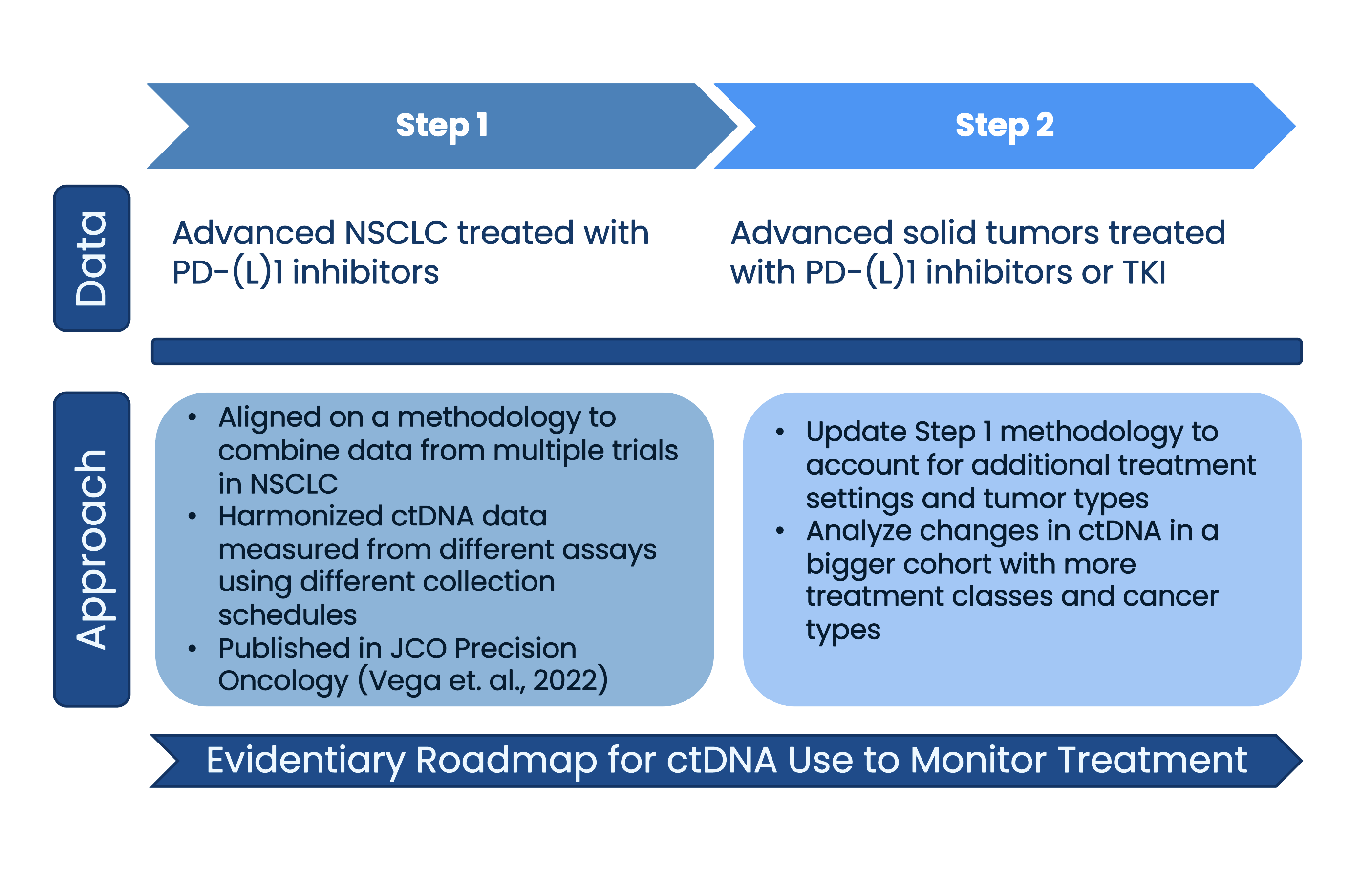
Step 1 of ctMoniTR aggregated five studies in patients with advanced non-small cell lung cancer (aNSCLC) treated with immunotherapy. Friends collaborated with our partners to identify these studies, create a plan to analyze the data, and perform the analysis. Findings showed robust and consistent associations between change in ctDNA levels and overall survival. Importantly, this work demonstrated that an initiative to harmonize clinical trials to measure ctDNA is possible.
Step 2 of the project expands the scope of this research to study the relationship between ctDNA and clinical outcomes across several clinical settings, drug classes, and cancer types. Initial findings from an analysis of 8 clinical trials of patients with aNSCLC treated with a tyrosine kinase inhibitor (TKI) build on Step 1 and shows that ctDNA clearance on treatment associates with improved overall survival.
Friends also leads a coordinated effort to apply findings from the ctMoniTR project and identify the evidence necessary to support use of ctDNA as an intermediate endpoint in drug development and regulatory decision-making.
Accelerated Approval allows effective treatments get to patients sooner and using ctDNA as an intermediate endpoint would reduce burdens on patients in clinical trials. ctDNA can be assessed through a blood draw, potentially allowing for measurement of a patient’s molecular response to therapy earlier, using a method that is less invasive for patients, and more frequently than radiographic response assessments. Moving an approach like this from concept to use in prospectively designed clinical trials would take substantially longer without Friends coordination and support from collaborative sponsors.
Project Overview
Project Outcomes
2025
- In April, a manuscript was published in Clinical Cancer Research, demonstrating that ctDNA clearance is associated with improved overall and progression-free survival in patients with advanced NSCLC treated with tyrosine kinase inhibitors (TKIs).
2024
- In May, a manuscript of findings from the Baseline ctDNA project was published in the journal Diagnostics.
- In November, Friends presented a poster at the Society for Immunotherapy in Cancer Annual Meeting.
- Friends discussed a framework for integrating change in ctDNA levels in advanced cancer clinical trials to support meta-analyses for intermediate endpoint validation during our Annual Meeting in 2024.
2023
- In June, Friends presented a poster at the ASCO Annual Meeting.
- In July, Friends hosted a meeting to share initial data from the Baseline ctDNA Project and results from ctMoniTR Step 2 Module 1 as well as discussed regulatory considerations for using ctDNA as an early endpoint.
2022
- In July, our working group presented the evidentiary roadmap in a public meeting discussing the use of ctDNA as an early endpoint.
- In October, Friends presented a poster at the 4th Annual ISLB Congress about the ctMoniTR Project.
- A manuscript of findings from ctMoniTR Step 1 was published in JCO Precision Oncology.
2021
- Step 2 of ctMoniTR launched.
- Friends discussed key considerations for expanding the work into early stage disease during our Annual Meeting in 2021.
2020
- We presented findings from ctMoniTR Step 1 in July.
- We held a question and answer session following the findings presentation in August. An event recap can be found here.
2019
- Friends launched the ctMoniTR Project.
2018
- The ctMoniTR project started following discussions at the Friends Annual Meeting 2018, which coincided with the release of a whitepaper.
Project Partners
ctMoniTR Step 1 Partners: AstraZeneca, Bristol Myers Squibb, Genentech, Inc., Guardant Health, Inc., Johns Hopkins University School of Medicine, LexentBio, Inc. (now Foundation Medicine, Inc.), Merck & Co., Inc., the NMD Group LLC, Penn Medicine, Roche Diagnostics, and the U.S. Food and Drug Administration (FDA)
ctMoniTR Step 2 Partners: Agilent Technologies, AstraZeneca, Bayer, Biodesix, Bio-Rad Laboratories, Inc., Boehringer Ingelheim, Bristol Myers Squibb Company, Cancer Research And Biostatistics (CRAB), Clovis Oncology, Inc., EMD Serono, Inc. (Merck KGaA), European Organization for Research and Treatment of Cancer (EORTC), Foundation Medicine, Inc., Genentech, Inc., Genmab, Guardant Health, Inc., Gritstone Bio, Inc., Illumina, Inc., Johns Hopkins University School of Medicine, Loxo@Lilly, MD Anderson Cancer Center, Molecular Characterization Laboratory (MoCha) at Frederick National Laboratory, Natera, Inc., NMD Group LLC, Novartis AG, Pfizer, Princess Margaret Cancer Centre, Regeneron Pharmaceuticals, Inc., Roche Diagnostics, Takeda Pharmaceutical Company, the U.S. Food and Drug Administration (FDA), and Xencor

