Two days before Christmas, the Food and Drug Administration gave Thomas Crawford an unexpected gift: approval of the first treatment ever for a devastating genetic disease that causes muscle wasting in babies and often results in death at an early age.
The drug “is nothing short of oh-my-God amazing” when given to infants who have not yet had symptoms, said Crawford, a Johns Hopkins pediatric neurologist who was involved in the clinical trials for the drug for spinal muscular atrophy.
What really surprised Crawford was the timing. The FDA approved Spinraza, as the treatment is called, in three months, five months ahead of its deadline. “That was very, very fast,” he said.
In President Trump’s first speech to Congress, he castigated the FDA for having a “slow and burdensome” approval process. At one point, he gestured toward a young woman in the gallery who has a different genetic disease, saying the agency’s sluggish pace blocked treatments from reaching desperately ill patients like her. He promised to “slash the restraints, not just at the FDA but across the government.”
Trump made similar remarks a few weeks ago when he met with pharmaceutical company executives at the White House. “You’re going to get your products either approved or not approved, but it’s going to be a quick process,” he told them. “It’s not going to take 15 years.”
Trump’s attacks have infuriated former and current agency officials who say they are unfair and misguided. They point out that drug reviews have sharply accelerated in recent decades. Today, the FDA routinely beats other regulators around the world and sometimes even gets heat for moving too quickly to green-light products.
The agency thus inhabits a kind of no man’s land — too fast and loose for some and too slow and rigid for others. Critics say it’s time to overhaul the way the place operates. But supporters worry about the damage that could be caused by a Trump-anointed commissioner who isn’t committed to the FDA’s mission of ensuring safety and effectiveness before drugs are marketed.
“The competitiveness of the U.S. pharmaceutical industry is based on the fact that the drug is validated as safe and effective by the FDA,” said David Kessler, who was the agency’s commissioner chief during the administrations of George H.W. Bush and Bill Clinton. “Any proposal that doesn’t require both will set back the industry’s competitiveness by 50 years.”
Kessler also noted that the first drug for Pompe disease — the ailment afflicting the young woman who attended Trump’s congressional address — was approved by the FDA in 2006 in nine months, based on a trial of a few dozen patients. “It’s the fastest and most efficient drug-review program in the world.”
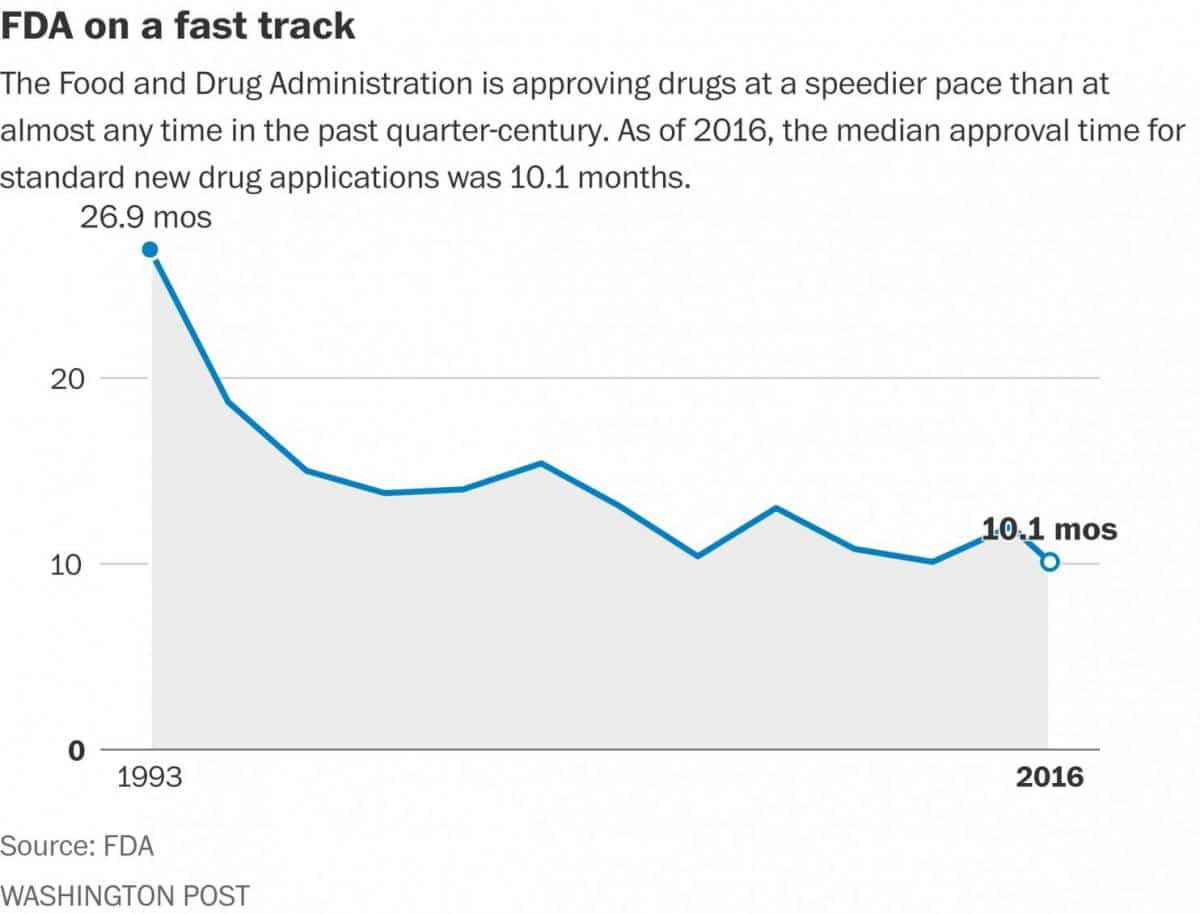
According to FDA figures, the median review time for drug approvals was 10 months in 2016, compared with 27 months in 1993. And for priority approvals — cases in which drugs show signs that they will be a marked improvement over existing treatments — the median time was eight months, compared with 20 months in 1993.
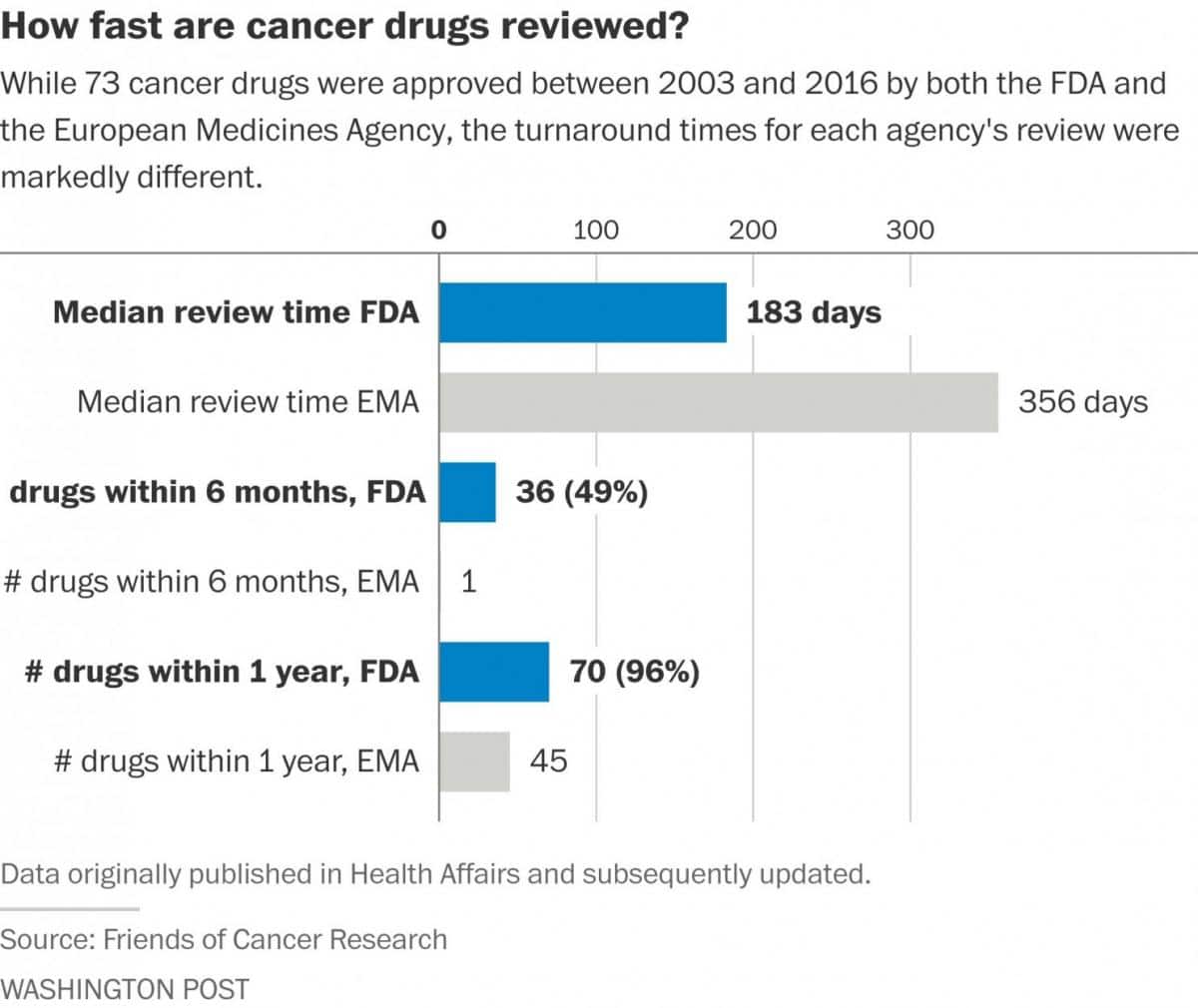
Cancer drugs are approved even faster — often within six months, according to data from the Friends of Cancer Research, an advocacy group. A comparison of 73 oncology drugs approved by both the FDA and the European Medicines Agency since 2003 showed the drugs were approved in half the time in the United States.
The FDA speed-up came after years of frustration among manufacturers and patient groups. It is the result of a decades-long effort from both Congress and the pharmaceutical companies regulated by the FDA to get more money, and thus more staff, for the agency. Under the Prescription Drug User Fee Act, which was first passed in 1992, for example, the agency collects hefty fees from drug manufacturers to fund the review process and, in turn, sets target deadlines for reviews.
The agency also has created expedited tracks for drugs that are especially promising or appear to treat an unmet medical need; in those cases, the deadlines are even tighter.
Some critics say all those changes mean the pendulum has swung too far in the industry’s direction. Michael Carome, director of Public Citizen’s Health Research Group, said the FDA is “promoting speed over the rigor of evidence” in judging products’ safety and effectiveness. And any move by the new administration to cut FDA regulations, he added, could lead to “countless preventable injuries, deaths and illnesses across the U.S.”
Vinay Prasad, an oncologist and assistant professor of medicine at the Oregon Health and Sciences University, agrees that FDA reviews should be tougher, not easier. He has repeatedly criticized the agency for approving cancer drugs that extend survival by just a few months, and he thinks Republican pressure will just make things worse.
“Republicans are obsessed with the idea that the FDA is a barrier to wonderful drugs reaching the market, but that isn’t true,” he said. “The barrier is that making wonderful drugs is scientifically difficult.”
But others say easing some FDA rules would be a boon to patients. Alden Abbott, deputy director of the Meese Center for Legal and Judicial Studies at the Heritage Foundation, said drug companies should be allowed to do limited marketing of drugs that have been shown to be safe in early-stage trials — and even before they have proved effective. “You need to ask whether the cost of waiting several years [while larger effectiveness trials are completed] are worth the extra time,” he said.
That view puts him in sync with comments made a few years ago by Jim O’Neill, an associate of Peter Thiel, the billionaire founder of PayPal who is an adviser to Trump. O’Neill, who has been widely mentioned as a possible FDA chief, gave a speech a few years ago in which he said that efficacy should be proved after drugs are on the market. Also reportedly in the running for the FDA job is Scott Gottlieb, a former deputy FDA commissioner who is considered a more traditional choice.
Stan Collender, a public relations executive who has a rare skin cancer and is being treated with immunotherapy, thinks the FDA has struck the right balance. “As a patient, I’ve got to tell you that moving ahead without safeguards makes me very, very nervous,” he said.
Groups that include industry representatives also praise the agency, especially for working with companies much earlier in the product-development process. “That’s one of the major changes over the last decade,” said Steven Grossman, a spokesman for the Alliance for a Stronger FDA, a coalition of industry officials and patient advocates who press for more funding for the agency. Edward Abrahams, president of the Personalized Medicine Coalition, praised the FDA for approving personalized therapies “in a timely manner.”
People inside and outside the industry agree that coming up with new drugs to present to the FDA is a long, expensive process. It can easily take 10 to 15 years develop a compound and conduct the trials needed for the initial FDA submission. The 21st Century Cures Act, which was passed last year, encourages the use of different types of evidence to move things more quickly — something that is both praised and criticized.
In Spinraza’s case, the FDA worked closely with the Biogen Inc., the drug developer. While spinal muscular atrophy is considered a rare disorder, it is the most common genetic cause of death among infants and young children. Babies who have the genetic defect are unable to achieve milestones such as sitting up, crawling and walking. Many die in childhood; most of the rest need around-the-clock care.
Gilmore O’Neill, Biogen’s senior vice president of late-stage clinical development, said the FDA worked closely with the company to figure out the best ways to design the clinical trial and test the drug’s effectiveness. It submitted its work on a rolling basis to speed things up.
Last August, when Biogen looked at the initial information emerging from a key clinical trial, the data was so good that it stopped the test so that the children in the placebo group could get the medicine. It submitted its application to the FDA a month later. Agency reviewers, said O’Neill, “really burned the midnight oil” to get the drug approved quickly — two days before Christmas.
Now that the drug is approved, its generating controversy because of its high price: $750,000 for the first year and $375,000 a year after that. Some insurers are restricting coverage to patients with certain types of the disease.
https://www.washingtonpost.com/news/to-your-health/wp/2017/03/02/trump-…
Click HERE to view the full updated comparison report of oncology approvals at the FDA vs. the EMA.