Although tumor mutational burden burden (TMB) is established as a clinically informative feature of tumors, its optimal use in therapeutic decision-making faces many challenges, and we are only beginning to fully understand its strengths and limitations. Development of biomarkers that can be readily applied in the clinic to identify patients most likely to respond to groundbreaking immunotherapies has become a priority in immuno-oncology.1
Next-generation sequencing (NGS), used to estimate the number of somatic mutations within a tumor specimen, is among the leading candidates for TMB detection. The pinnacle of its success arrived in mid-2020 with the tumor agnostic FDA approval of the immune checkpoint inhibitor (ICI) pembrolizumab (Keytruda) for patients with high TMB.2 New studies evaluating the pancancer role of TMB as a predictive biomarker of response to ICIs have revealed some of its limitations, raising doubts about its broad applicability and the appropriateness of a universal TMB cutoff.3-6
In non–small cell lung cancer (NSCLC), the predictive power of TMB is perhaps best characterized, these challenges present a hurdle to clinical implementation of TMB assays. A supplemental biologics license application for the dual checkpoint inhibition in patients with NSCLC who had high TMB was subsequently withdrawn by the drug developers when a phase 3 trial failed to demonstrate an overall survival (OS) benefit in this population.7,8 Ongoing research is focused on finding ways to unleash the true potential of TMB, including efforts to standardize its measurement, define better cutoffs, and understand its role in common cancer types that have been largely overlooked.7,8
Ongoing research is focused on finding ways to unleash the true potential of TMB, including efforts to standardize its measurement, define better cut-offs, and understand its role in common cancer types that have been largely overlooked to date.9,10
The More the Merrier?
In 2017, pembrolizumab received an historic tumor agnostic approval for the treatment of tumors with high levels of microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR) after these biomarkers were shown to identify patients with improved outcomes.11 The theory behind the efficacy of ICIs in this patient population was that tumors with greater numbers of mutations provoke a stronger antitumor immune response because they increase the presence of neoantigens, which activate cytotoxic T cells, on the tumor cell surface.9,12,13
Mismatch repair (MMR) is a mechanism through which cells repair types of DNA damage that typically occur within areas of short, repetitive DNA sequences known as microsatellites. Abnormalities in the MMR pathway lead to inability to repair damaged DNA and accumulation of mutations, as well as a characteristic variability in the lengths of microsatellites; thus, MSI-H is an indication of dMMR.14
Although dMMR/MSI-H is more common in some tumors than others, it is observed in a small proportion of patients overall.15 Although almost all tumors with MSI-H have high mutation rates, less than one-fifth of highly mutated tumors display MSI-H, suggesting that other mechanisms drive some tumors to acquire more mutations.16
Assessment of TMB using whole-exome sequencing (WES) allowed investigators to capture highly mutated tumors irrespective of cause; although TMB varies, a subset of patients with high TMB can be observed in almost every cancer type.16 Initial retrospective studies in NSCLC and melanoma demonstrated that higher levels of TMB were associated with improved outcomes following ICIs.17,18 Since then, TMB has emerged as a powerful predictor of response to ICIs across different cancer types.3,9
Targeted NGS panels, which are faster and less expensive than WES, provide a comparable estimate of TMB and are widely used.19-21 Most measure TMB from tissue biopsies, but liquid biopsies that use circulating tumor DNA isolated from the blood are being developed.9
A growing number of prospective trials have been initiated to validate the relationship between TMB and ICI outcomes. Most notable is the phase 2 KEYNOTE-158 trial (NCT02628067), in which pembrolizumab monotherapy was evaluated in patients with multiple types of advanced solid tumors that had progressed following prior treatment. Among 102 patients with high levels of TMB (≥ 10 mutations [mut]/Mb), the overall response rate (ORR) was 29.4% (95% CI, 20.8%-39.3%), with complete responses in 4 patients and 57% of responses lasting at least 12 months. Responses were observed across 8 tumor types. An ORR of 6.3% (95% CI, 4.6%8.3%) was observed in patients with TMB lower than 10 mut/Mb (n = 688).2
These results led the FDA to approve pembrolizumab in June 2020 for the treatment of adult and pediatric patients with unresectable or metastatic TMB-high (≥ 10 mut/Mb) solid tumors, securing another tumor agnostic win for the drug. The FoundationOne CDx assay, a 324-gene panel that estimates TMB based on a 0.8 Mb region of the exome, was approved as a companion diagnostic for use in this indication.2
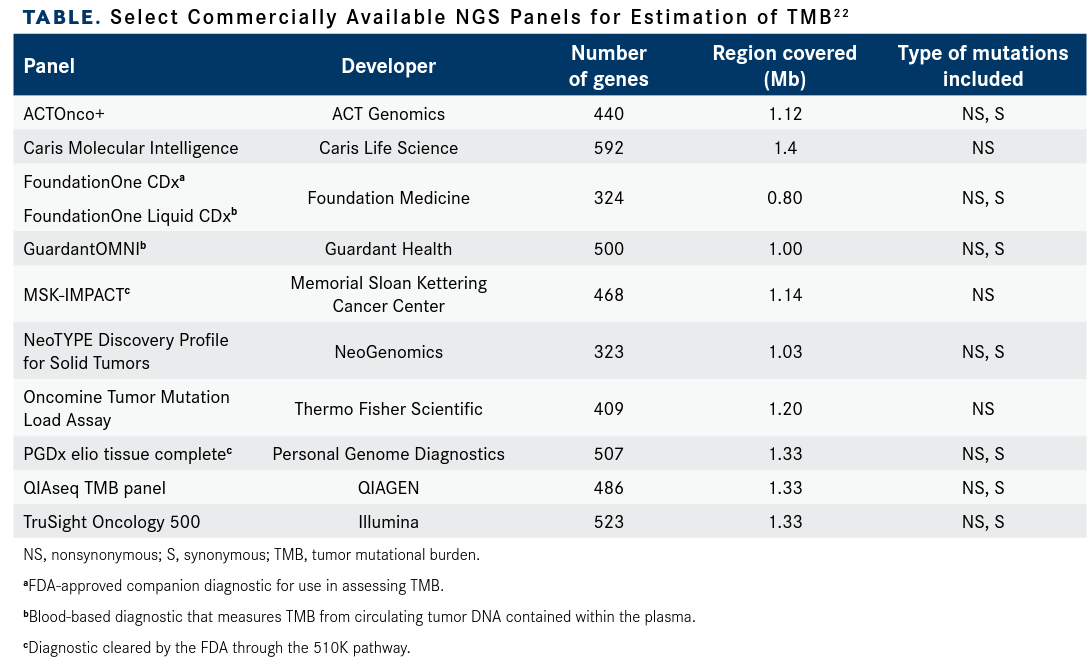
Although FoundationOne CDx is the only FDA-approved TMB assay, Memorial Sloan Kettering Cancer Center in New York, New York, developed MSK-IMPACT, which leverages a 468-gene panel covering a 1.1-Mb region of the exomet and has been authorized by the FDA through the 510K pathway. Numerous other TMB assays are commercially available for use in a research setting (TABLE).22
The abundance of available assays has led to questions surrounding standardization of TMB estimation across different assays and tumor types. Friends of Cancer Research established a TMB Harmonization Consortium of stakeholders from pharmaceutical and diagnostic companies and scientific organizations, including the FDA, to conduct a comprehensive review of available data regarding TMB and response to immunotherapy.
Although tumor mutational burden burden (TMB) is established as a clinically informative feature of tumors, its optimal use in therapeutic decision-making faces many challenges, and we are only beginning to fully understand its strengths and limitations. Development of biomarkers that can be readily applied in the clinic to identify patients most likely to respond to groundbreaking immunotherapies has become a priority in immuno-oncology.1
Next-generation sequencing (NGS), used to estimate the number of somatic mutations within a tumor specimen, is among the leading candidates for TMB detection. The pinnacle of its success arrived in mid-2020 with the tumor agnostic FDA approval of the immune checkpoint inhibitor (ICI) pembrolizumab (Keytruda) for patients with high TMB.2 New studies evaluating the pancancer role of TMB as a predictive biomarker of response to ICIs have revealed some of its limitations, raising doubts about its broad applicability and the appropriateness of a universal TMB cutoff.3-6
In non–small cell lung cancer (NSCLC), the predictive power of TMB is perhaps best characterized, these challenges present a hurdle to clinical implementation of TMB assays. A supplemental biologics license application for the dual checkpoint inhibition in patients with NSCLC who had high TMB was subsequently withdrawn by the drug developers when a phase 3 trial failed to demonstrate an overall survival (OS) benefit in this population.7,8 Ongoing research is focused on finding ways to unleash the true potential of TMB, including efforts to standardize its measurement, define better cutoffs, and understand its role in common cancer types that have been largely overlooked.7,8
Ongoing research is focused on finding ways to unleash the true potential of TMB, including efforts to standardize its measurement, define better cut-offs, and understand its role in common cancer types that have been largely overlooked to date.9,10
The More the Merrier?
In 2017, pembrolizumab received an historic tumor agnostic approval for the treatment of tumors with high levels of microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR) after these biomarkers were shown to identify patients with improved outcomes.11 The theory behind the efficacy of ICIs in this patient population was that tumors with greater numbers of mutations provoke a stronger antitumor immune response because they increase the presence of neoantigens, which activate cytotoxic T cells, on the tumor cell surface.9,12,13
Mismatch repair (MMR) is a mechanism through which cells repair types of DNA damage that typically occur within areas of short, repetitive DNA sequences known as microsatellites. Abnormalities in the MMR pathway lead to inability to repair damaged DNA and accumulation of mutations, as well as a characteristic variability in the lengths of microsatellites; thus, MSI-H is an indication of dMMR.14
Although dMMR/MSI-H is more common in some tumors than others, it is observed in a small proportion of patients overall.15 Although almost all tumors with MSI-H have high mutation rates, less than one-fifth of highly mutated tumors display MSI-H, suggesting that other mechanisms drive some tumors to acquire more mutations.16
Assessment of TMB using whole-exome sequencing (WES) allowed investigators to capture highly mutated tumors irrespective of cause; although TMB varies, a subset of patients with high TMB can be observed in almost every cancer type.16 Initial retrospective studies in NSCLC and melanoma demonstrated that higher levels of TMB were associated with improved outcomes following ICIs.17,18 Since then, TMB has emerged as a powerful predictor of response to ICIs across different cancer types.3,9
Targeted NGS panels, which are faster and less expensive than WES, provide a comparable estimate of TMB and are widely used.19-21 Most measure TMB from tissue biopsies, but liquid biopsies that use circulating tumor DNA isolated from the blood are being developed.9
A growing number of prospective trials have been initiated to validate the relationship between TMB and ICI outcomes. Most notable is the phase 2 KEYNOTE-158 trial (NCT02628067), in which pembrolizumab monotherapy was evaluated in patients with multiple types of advanced solid tumors that had progressed following prior treatment. Among 102 patients with high levels of TMB (≥ 10 mutations [mut]/Mb), the overall response rate (ORR) was 29.4% (95% CI, 20.8%-39.3%), with complete responses in 4 patients and 57% of responses lasting at least 12 months. Responses were observed across 8 tumor types. An ORR of 6.3% (95% CI, 4.6%8.3%) was observed in patients with TMB lower than 10 mut/Mb (n = 688).2
These results led the FDA to approve pembrolizumab in June 2020 for the treatment of adult and pediatric patients with unresectable or metastatic TMB-high (≥ 10 mut/Mb) solid tumors, securing another tumor agnostic win for the drug. The FoundationOne CDx assay, a 324-gene panel that estimates TMB based on a 0.8 Mb region of the exome, was approved as a companion diagnostic for use in this indication.2
Although FoundationOne CDx is the only FDA-approved TMB assay, Memorial Sloan Kettering Cancer Center in New York, New York, developed MSK-IMPACT, which leverages a 468-gene panel covering a 1.1-Mb region of the exomet and has been authorized by the FDA through the 510K pathway. Numerous other TMB assays are commercially available for use in a research setting (TABLE).22
The abundance of available assays has led to questions surrounding standardization of TMB estimation across different assays and tumor types. Friends of Cancer Research established a TMB Harmonization Consortium of stakeholders from pharmaceutical and diagnostic companies and scientific organizations, including the FDA, to conduct a comprehensive review of available data regarding TMB and response to immunotherapy.
They found that some assays consistently underestimated TMB and others overestimated, compared with WES-derived TMB, with the size and content of the gene panel identified as key factors in assay performance. They developed consensus recommendations for standardization of NGS panels for TMB estimation and built a calibration tool to enhance the comparability of assays.23,24
Potential Pitfalls
Doubts remain regarding the broad applicability of TMB as a predictive biomarker and over the use of a universal cutoff for determining high TMB. A criticism of KEYNOTE-158 is that it did not include more common tumor types, such as colorectal cancer, prostate, and breast cancer.10
The predictive role of TMB is being explored in these settings. Prostate cancer is notable considering that ICIs typically offer little benefit to most patients. Preliminary data presented at the American Society of Clinical Oncology Genitourinary Cancers Symposium demonstrated that a cohort of patients with advanced prostate cancer who received ICI therapy guided by blood-based TMB score experienced clinical benefit.25 In a separate real-world biomarker study, investigators showed that patients with metastatic castration-resistant prostate cancer who had high TMB (≥ 10 mut/Mb) derived greater benefit from ICIs vs taxane chemotherapy.26
Another issue with KEYNOTE-158 is that among the tumor types enrolled, TMB appeared to be a better predictor of response in only some tumors. For example, the ORR in patients with endometrial cancer was 47%, compared with 7% in patients with anal cancer.2
Additionally, the study measured ORR and not more clinically meaningful end points. Several subsequent studies have now evaluated the relationship between patient survival and TMB. Samstein et al analyzed the clinical and genomic data of 1662 patients with advanced cancer treated with ICIs whose tumors were evaluated for TMB using the MSK-IMPACT assay. High TMB (defined as the highest 20% in each tumor type) was associated with improved OS across most cancer types, although in some cases not statistically significantly.3
A notable exception was glioma, in which high TMB was associated with poorer survival,3 possibly because the high TMB observed in glioma may result from treatment with DNA-damaging cytotoxic drugs and may not necessarily be associated with increased infiltration of T cells into the tumor microenvironment. Another study demonstrated an association between TMB and improved OS following ICI therapy, but only among tumors in which the levels of CD8-positive T cells correlated with predicted neoantigen load, such as melanoma, lung cancer, and bladder cancer.5
In a third study examining the relationship between TMB and post-ICI OS, which used the approved cutoff of 10 mut/Mb, the median OS was longer only in patients with certain types of MMR-proficient cancer, such as head and neck cancer, NSCLC, and melanoma, with no benefit in patients with other cancer types.4 These results have led some to argue that the clinical benefit of high TMB as a biomarker has not been convincingly demonstrated across all tumor histologies.10
Another challenge to the clinical application of TMB assays is how to define an appropriate cutoff for high TMB. In their study, Samstein et al found that the top 20% of TMB varied markedly between cancer types.3 In another study, investigators sought to determine how the FDA-approved cut-off performed in a realworld setting. They analyzed the outcomes of more than 1600 patients treated with ICIs.
Overall patients with a TMB of at least 10 mut/Mb had better outcomes than those with a TMB below 10 mut/Mb; the universal cutoff did not reliably predict increased survival after immunotherapy across all tumor types. Notably, in patients with renal cell carcinoma, no cases met the high TMB threshold but many patients still responded.6 Many argue that a cancer type-specific threshold may be better, whereas others have suggested that an arbitrary threshold of high TMB is wholly inappropriate and that the use of ICIs should instead be considered in the context of the cause of the TMB.4,6
TMB in NSCLC
The greatest preponderance of data showing an association between TMB and ICI response comes from NSCLC, but even in this setting its predictive role remains uncertain. Subgroup analyses from randomized clinical trials of ICIs in NSCLC in various settings demonstrated significantly improved response rates and progression-free survival (PFS) in patients with high TMB compared with those with low TMB when ICIs were used as monotherapy.27,28
However, its predictive role in patients receiving ICIs in combination with chemotherapy appears more limited; in the KEYNOTE-189 (NCT02578680) study, high TMB was not significantly associated with improved OS, PFS or ORR in patients treated with pembrolizumab plus chemotherapy as first-line therapy for metastatic nonsquamous NSCLC.29
Foundation Medicine’s blood-based TMB assay was being used in the phase 2/3 B-F1RST (NCT02848651) and phase 3 B-FAST (NCT03178552) trials, which evaluated the potential of TMB as a predictive biomarker for atezolizumab (Tecentriq) in the frontline treatment of advanced/metastatic NSCLC. High TMB (defined as 14.5 mut/Mb) was measured as 16 or greater in the blood TMB (bTMB) scale.
InB-F1RST, the primary biomarker end point was investigator-assessed PFS at the cutoff of bTMB of at least 16. The median PFS was 5 months (95% CI, 1.6-10.8) in patients with high TMB compared with 3.5 months (95% CI, 2.6-4.3) for those with low TMB (HR, 0.80; 90% CI, 0.541.18; P = .35). The median OS was 23.9 months (95% CI, 8.8-not estimable) vs 13.4 months (HR, 0.66; 90% CI, 0.40-1.10; P = .18), respectively. Further, the ORR in the bTMB-high group was 35.7% (95% CI, 19.2%-55.5%) vs 5.5% in the bTMB-low group (95% CI, 2.2%-12.2%) (P < .0001).30 In B-FAST, in which atezolizumab monotherapy was compared with platinum-based chemotherapy in biomarker-selected patients with advanced NSCLC, results from cohort C, in which patients were selected based upon high bTMB levels, failed to meet the primary end point of PFS.31
References
1. Bai R, Lv Z, Xu D, Cui J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomarker Res. 2020;8(1):34. doi:10.1186/s40364-020-00209-0
2. Marcus L, Fashoyin-Aje LA, Donoghue M, et al. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin Cancer Res. 2021;27(17):4685-4689. doi:10.1158/1078-0432.CCR-21-0327
3. Samstein RM, Lee CH, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202-206. doi:10.1038/s41588-018-0312-8
4. Rousseau B, Foote MB, Maron SB, et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N Engl J Med. 2021;384(12):1168-1170. doi:10.1056/NEJMc2031965
5. McGrail DJ, Pilié PG, Rashid NU, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661-672. doi:10.1016/j.annonc.2021.02.006
6. Valero C, Lee M, Hoen D, et al. The association between tumor mutational burden and prognosis is dependent on treatment context. Nat Genet. 2021;53(1):11-15. doi:10.1038/s41588-020-00752-4
7. Nawrat A. BMS withdraws US sBLA for Opdivo-Yervoy combination for NSCLC. Pharmaceutical Technology. Updated January 28, 2019. Accessed August 1, 2022. bit.ly/3SPsD2w
8. U.S. Food and Drug Administration (FDA) accepts application for Opdivo plus low-dose Yervoy for treatment of first-line non-small cell lung cancer in patients with tumor mutational burden ≥10 mut/Mb. News release. Bristol Myers Squibb. June 21, 2018. Accessed August 1, 2022. bit.ly/3AocOc7
9. Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. 2021;39(2):154-173. doi:10.1016/j.ccell.2020.10.001
10. Strickler JH, Hanks BA, Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: is more always better? Clin Cancer Res. 2021;27(5):1236-1241. doi:10.1158/1078-0432.CCR-20-3054
11. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. FDA. Updated May 30, 2017. Accessed August 1, 2022. bit.ly/3QFpsbM
12. Klempner SJ, Fabrizio D, Bane S, et al. Tumor mutational burden as a predictive biomarker for response to immune checkpoint inhibitors: a review of current evidence. Oncologist. 2020;25(1):e147-e159. doi:10.1634/theoncologist.2019-0244
13. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1-2):48-61. doi:10.1016/j.cell.2014.12.033
14. Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22(4):813-820. doi:10.1158/1078-0432.CCR-15-1678
15. Lorenzi M, Amonkar M, Zhang J, Mehta S, Liaw KL. Epidemiology of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in solid tumors: a structured literature review. J Oncol. 2020;2020:e1807929. doi:10.1155/2020/1807929
16. Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi:10.1186/s13073-017-0424-2
17. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189-2199. Published correction appears in N Engl J Med. 2018;379(22):2185.
18. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348(6230):124-128. doi:10.1126/science.aaa1348
19. Campesato LF, Barroso-Sousa R, Jimenez L, et al. Comprehensive cancer-gene panels can be used to estimate mutational load and predict clinical benefit to PD-1 blockade in clinical practice. Oncotarget. 2015;6(33):34221-34227. doi:10.18632/oncotarget.5950
20. Johnson DB, Frampton GM, Rioth MJ, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res. 2016;4(11):959-967. doi:10.1158/2326-6066.CIR-16-0143
21. Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633-641. doi:10.1200/JCO.2017.75.3384
22. Sholl LM, Hirsch FR, Hwang D, et al. The promises and challenges of tumor mutation burden as an immunotherapy biomarker: a perspective from the International Association for the Study of Lung Cancer Pathology Committee. J Thorac Oncol. 2020;15(9):1409-1424. doi:10.1016/j.jtho.2020.05.019
23. Vega DM, Yee LM, McShane LM, et al. Aligning tumor mutational burden (TMB) quantification across diagnostic platforms: phase II of the Friends of Cancer Research TMB Harmonization Project. Ann Oncol. 2021;32(12):1626-1636. doi:10.1016/j.annonc.2021.09.016
24. Merino DM, McShane LM, Fabrizio D, et al. Establishing guidelines to harmonize tumor mutational burden (TMB): in silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8(1):e000147. doi:10.1136/jitc-2019-000147
25. Rauterkus G, Hadadi A, Barnett R, et al. Blood-based tumor mutational burden from circulating tumor DNA and immune checkpoint inhibitors in advanced prostate cancer. J Clin Oncol. 2022;40(suppl 6):165-165. doi:10.1200/JCO.2022.40.6_suppl.165
26. Sayegh N, Graf R, Fisher V, et al. Tumor mutational burden as a predictive biomarker for immune checkpoint inhibitor versus taxane chemotherapy benefit in metastatic castration-resistant prostate cancer: a real-world biomarker study. J Clin Oncol. 2022;40(suppl 6):162-162. doi:10.1200/JCO.2022.40.6_suppl.162
27. Galvano A, Gristina V, Malapelle U, et al. The prognostic impact of tumor mutational burden (TMB) in the first-line management of advanced non-oncogene addicted non-small-cell lung cancer (NSCLC): a systematic review and meta-analysis of randomized controlled trials. ESMO Open. 2021;6(3):100124. doi:10.1016/j.esmoop.2021.100124
28. Meng G, Liu X, Ma T, Lv D, Sun G. Predictive value of tumor mutational burden for immunotherapy in non-small cell lung cancer: a systematic review and meta-analysis. PLoS ONE. 2022;17(2):e0263629. doi:10.1371/journal.pone.0263629
29. Garassino M, Rodriguez-Abreu D, Gadgeel S, et al. OA04.06 Evaluation of TMB in KEYNOTE-189: pembrolizumab plus chemotherapy vs placebo plus chemotherapy for nonsquamous NSCLC. J Thorac Oncol. 2019;14(10):S216-S217. doi:10.1016/j.jtho.2019.08.427
30. Kim ES, Velcheti V, Mekhail T, et al. Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: the phase 2 B-F1RST trial. Nat Med. 2022;28(5):939-945. doi:10.1038/s41591-022-01754-x
31. Dziadziuszko R, Peters S, Gadgeel S. Atezolizumab (atezo) vs platinum-based chemo in blood-based tumor mutational burden-positive (bTMB+) patients (pts) with first-line (1L) advanced/metastatic (m)NSCLC: results of the Blood First Assay Screening Trial (BFAST) phase III cohort C. Ann Oncol. 2021;32(suppl 5):S950-S951. doi:10.1016/j.annonc.2021.08.1883
https://www.onclive.com/view/tumor-agnostic-role-of-tmb-biomarker-faces-challenges
