New findings by Friends of Cancer Research (Friends) were published recently in JCO Clinical Cancer Informatics “Evaluation of real-world tumor response derived from electronic health record data sources: A feasibility analysis in mNSCLC patients treated with chemotherapy.” This salient publication demonstrates the feasibility of aligning disparate real-world (rw) data sources to evaluate rw-response to treatment using clinician-documented response in patients with metastatic non-small cell lung cancer (mNSCLC).
The publication builds on a series of collaborative real-world evidence (RWE) projects in Friends Real-World Evidence Portfolio which establishes pre-specified methodological approaches to assess the feasibility of using real-world data (RWD) to evaluate benefit for patients.
“The publication provides valuable insights into the availability of data to assess rw-response and supports the use of clinician-stated response. The demonstrated feasibility and relative consistency across data providers in rw-endpoints suggests further exploration may inform drug effectiveness evaluation,” said Dr. Brittany Avin McKelvey, Director of Regulatory Affairs at Friends.
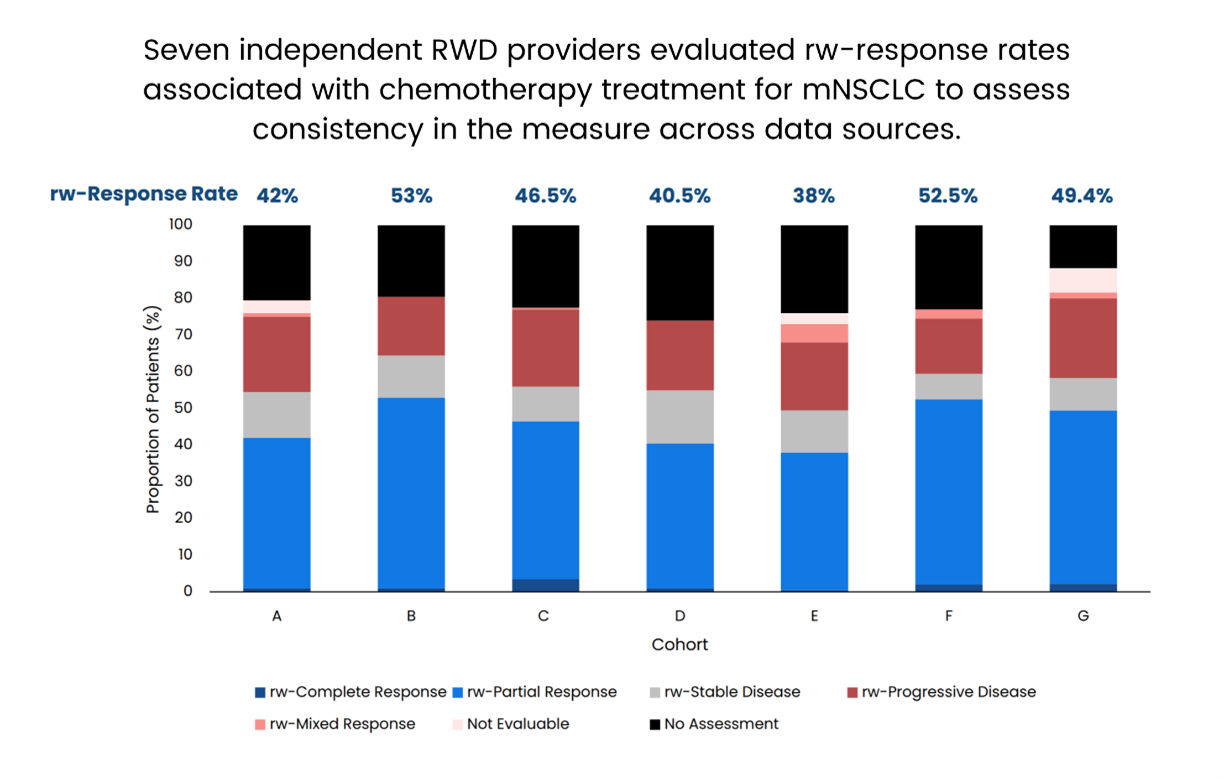
“Response rate is essential in cancer drug development, and rw-response could have a potentially valuable role in identifying effectiveness signals within RWD sources for new indications or elucidating treatment outcomes in underrepresented populations,” stated Donna R. Rivera, Associate Director for Pharmacoepidemiology in the FDA Oncology Center of Excellence and leader of the Oncology RWE Program. “Additional work is needed to prospectively standardize real-world endpoint definitions toward the interconnected goal of building learning health systems.”
This research is based on a collaborative effort across RWD providers, pharmaceutical companies, academics, researchers, and experts from government agencies to develop and implement a common protocol and statistical analysis plan. Aligning methodologies for aggregating and analyzing RWD will support its use as a reliable and consistent source to generate RWE to support drug development and regulatory decision-making.
To learn more about Friends RWE portfolio and the projects, events and findings that have led to this important publication, click here.
Manuscript Authors
Brittany A McKelvey, Elizabeth Garrett-Mayer, Donna R Rivera, Amy Alabaster, Hillary S Andrews, Elizabeth G Bond, Thomas D Brown, Amanda Bruno, Lauren Damato, Janet L Espirito, Laura L Fernandes, Eric Hansen, Paul Kluetz, Xinran Ma, Andrea McCracken, Pallavi S Mishra-Kalyani, Yanina Natanzon, Danielle Potter, Nicholas J Robert, Lawrence Schwartz, Regina Schwind, Connor Sweetnam, Joseph Wagner, Mark D Stewart, Jeff D Allen.
Manuscript Partners
American Society of Clinical Oncology, ConcertAI, COTA, Flatiron Health, Friends of Cancer Research, Guardian Research Network, IQVIA, MSK, Ontada, Inc., Syapse, Syneos Health, Tempus AI, US FDA.
About Friends of Cancer Research
Friends of Cancer Research (Friends) is working to accelerate policy change, support groundbreaking science, and deliver new therapies to patients quickly and safely. We unite scientists, industry researchers, patient advocates, and policy makers to build unique collaborations able to instigate progress faster than any one organization working alone. This collaboration among partners from every healthcare sector ultimately drives advances in science, policy, and regulation that speed life-saving treatments to patients.
For more information, please visit https://friendsofcancerresearch.org/.
To subscribe to Friends emails click here.

