ECA Pilot Project
Informing the Use of External Control Arms in Clinical Development and Regulatory Decision-Making
- Traditional randomized controlled trials (RCTs) are the gold standard for evaluating new therapies but in many rare diseases and cancers, they are not always feasible or ethical. Small patient populations make it difficult to enroll enough trial participants for robust statistical analysis. In some cases, it can be ethically complex to randomize patients to a control arm, particularly when effective options are limited or nonexistent.
- This creates a critical need for reliable alternative approaches that generate scientifically valid and meaningful data. Without solutions to overcome these challenges, there may be insufficient evidence to make informed decisions about new therapies and advance the development of potentially life-saving treatments for patients. This contributes to delays in patient access and hinders innovation in areas of high unmet need, particularly for populations affected by rare or aggressive diseases.
- The External Control Arm (ECA) pilot project builds upon Friends’ RWE Portfolio to advance oncology clinical research and regulatory decision-making. Through leveraging insights from past RWD initiatives, this project will identify and refine best practices for using historical clinical trial data and RWD to construct rigorous ECAs or augment single arm trials.
- Since 2017, Friends has led collaborations between RWD developers, industry, government agencies, and academic researchers to address challenges in using RWD/E for clinical development. This project builds on prior initiatives—RWE Pilot 1.0, Pilot 2.0, rw-Response, and rw-Care—which advanced methods for endpoint alignment, trial emulation, real-world response measurement, and clinical decision-making. Visit our RWE portfolio site for additional details.
- Developing effective and ethical approaches to clinical research can significantly benefit patients, especially those with rare or hard-to-treat conditions where RCTs are challenging or impractical. Using RWD and historical clinical trial data to create an ECA has the potential to accelerate evidence generation and regulatory decision-making for promising therapies, bringing them to patients faster.
- In situations where traditional RCTs are not possible, ECAs provide a critical pathway for evaluating and approving new treatments, ensuring those with urgent medical needs gain access to life-saving options sooner. Use of external data also has the potential to provide additional context for single-arm studies. Additionally, they have the potential to augment traditional control arms to enable greater access to the experimental treatment for trial participants.
- Friends’ collaborative approach and the support of our partners will align best practices for using RWD or historical clinical trial data to construct ECAs, enabling evidence generation and the delivery of innovative treatments to patients quickly and safely.
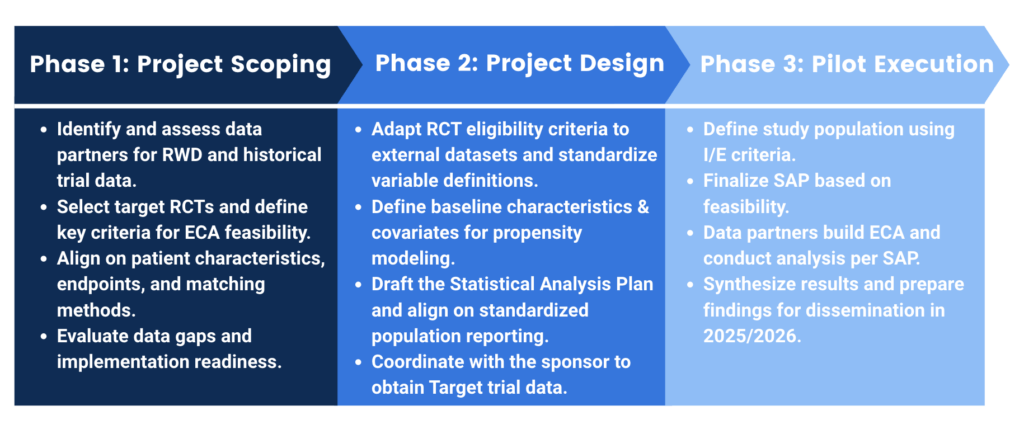
Project Timeline
Project Partners
ECA Pilot Project: AbbVie, Aetion, American Society of Clinical Oncology (ASCO), AstraZeneca, BeOne Medicines USA, Bristol Myers Squibb (BMS), ConcertAI, COTA, Eisai, Flatiron Health, Gemini, Genentech, IQVIA/ GRN, Johnson and Johnson Innovative Medicine, Medidata Solutions, Merck, N-Power Medicine, Ontada/McKesson, Pancreatic Cancer Action Network (PanCAN), Tempus, and Truveta.

