Digital PATH Project
Digital and Computational Pathology Tool Harmonization Project
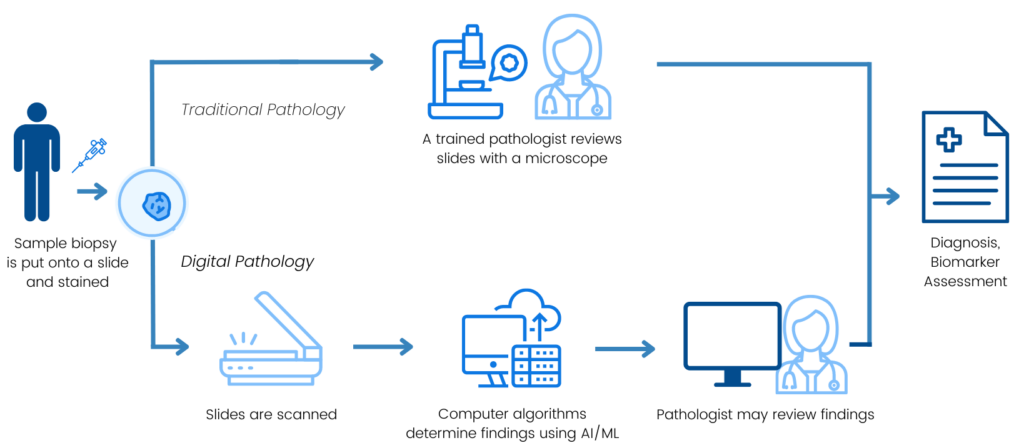
Traditionally, pathologists examine tissue obtained through a biopsy to diagnose cancer, determine the type and stage, and identify biomarkers that not only indicate a patient’s likely response to certain treatments but also provide other clinical insights. Digital pathology enables innovative approaches to these assessments through the scanning and digitization of slides for storage, viewing, and analysis. Sometimes, analyses can include the use of computational pathology platforms with artificial intelligence (AI)/machine learning (ML) algorithms to aid the pathologist in tissue analysis.
Similar to previous Friends’ efforts assessing sources of variability in biomarker outputs to identify opportunities to harmonize methodology to support aligned measurement and use (i.e., the TMB Harmonization Project and the HRD Harmonization Project), Friends convened a group of algorithm developers, patient advocates, government officials, pathologists, and drug developers to assess the comparability of biomarker measurements across computational pathology platforms, identify factors that may contribute to any observed variability, and propose areas for alignment.
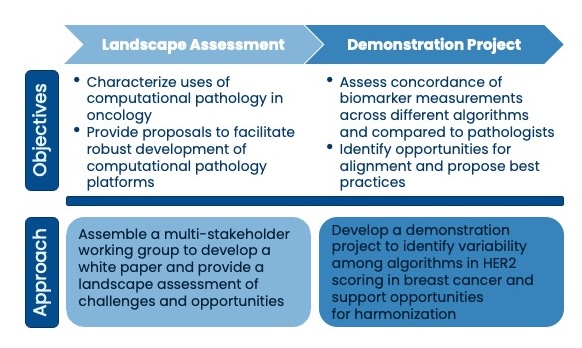
Friends first assembled a multi-stakeholder group to identify opportunities for using digital and computational pathology in oncology drug development. The group evaluated existing regulatory frameworks and developed proposals to support robust development of these emerging technologies including a risk-based approach to assessing evidentiary needs for validation.
The multi-stakeholder working group is evaluating HER2 assessments in breast cancer across multiple computational pathology platforms to understand the level of variability across digital and computational pathology platforms.
Digital and computational pathology platforms have the potential to provide greater accuracy, reproducibility, and standardization of pathology features, expedite diagnosis or pathological scoring, establish new biomarkers, and identify and select the appropriate patients for treatments – all of which can contribute to improving patient outcomes. Supporting the robust development of these platforms and identifying potential sources of variability will help to inform future use and advancements in technology that deliver more precise patient care. Without Friends coordination and support from collaborative sponsors, groups may never align on a solution to improve consistency across assays and interpreting results would be more challenging for patients and providers.
Project Overview
Project Outcomes
2025
- Digital PATH project findings were presented at Friends’ public meeting.
- Friends discussion document highlighting key considerations for establishing a reference dataset to validate digital pathology tools was published ahead of Friends’ public meeting.
2024
- Friends launched a demonstration project to evaluate how comparable HER2 biomarker assessments are across computational pathology models with a common set of breast cancer samples. This work will help to identify factors that may contribute to variability and propose alignment in the field.
- Experts discussed the objectives of the demonstration project during Friends’ public meeting.
- In December, Friends presented a poster at the San Antonio Breast Cancer Symposium.
2023
- Friends worked with stakeholders to characterize current and future uses of digital and computational pathology platforms and AI/ML in oncology drug development and reported findings in a white paper.
- Friends hosted a virtual public meeting to explore future opportunities to leverage existing data to support the proposals identified in the white paper.
Project Partners
Digital PATH Project: 4D 4D Path Inc., Amgen, AstraZeneca, BostonGene, Bristol Myers Squibb, Caris Life Sciences, Daiichi Sankyo, EMD Serono, Inc., Emory University, GA Green Consulting LLC, GSK, Indica Labs, Johnson and Johnson Innovative Medicine, Karolinska Institutet, Kulig Consulting, Loxo@Lilly, Lunit, Molecular Characterization Laboratory (MoCha) at Frederick National Laboratory, MD Anderson Cancer Center, Merck and Co., inc, National Cancer Institute (NCI), Nucleai, Panakeia, PathAI, Patient Advocates, Roche Diagnostics, Sanofi, Tempus AI, Inc., the U.S. Food and Drug Administration (FDA), ZAS Hospital, University of North Carolina, and Verily.
Landscape Assessment: 4D Path Inc., Amgen, AstraZeneca, Bristol Myers Squibb, EMD Serono (Merck KGaA), GlaxoSmithKline, Kulig Consulting, Loxo@Lilly, Massachusetts General Hospital, MD Anderson Cancer Center, Merck & Co., Inc., Neomorph, Inc., Paige AI, PathAI, Sanofi, Tempus Labs, Inc., the U.S. Food and Drug Administration (FDA), University Hospital of Antwerp, University of North Carolina at Chapel Hill.

