
Welcome to Friends’ first Quarterly Patient Advocate Newsletter of 2024.
Here’s what you can read more about in this newsletter:
- What’s Happening at Friends?: Read about Friends’ research to ensure diagnostic tests consistently provide reliable results for patients and providers.
- Mark Your Calendars: Upcoming events, including public meetings hosted by Friends and other relevant stakeholders.
- Important News in Cancer Research: Recent news, FDA activities, and policy developments highlighting topics related to Friends’ work and topics impacting cancer care and drug development.
- Did You Know?: Learn more about how our harmonization projects fit into recent proposals for modernized diagnostic regulation.
- In Case You Missed It: Recaps of recent events and links to new publications and content from Friends.
What's Happening at Friends in 2024?
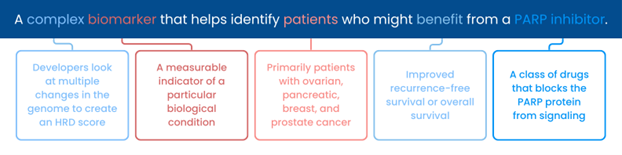
Diagnostic testing plays an important role in oncology care and treatment decision-making, including identifying whether a patient has a specific biomarker that may respond to targeted therapy. Often, several diagnostic tests that look for the same biomarker are available to patients and providers, and each test may use a different approach (e.g., different values or thresholds that determine the result) to identify the biomarker. These differences in test characteristics and approaches can lead to variability in results that may impact treatment decision-making and, ultimately, patient outcomes.
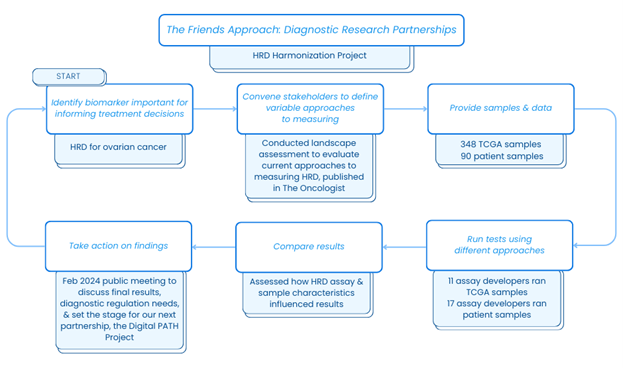
Friends’ diagnostic research partnerships assess variability across diagnostic tests for certain biomarkers and identify areas for alignment. The results of our most recent diagnostic research partnership, the HRD Harmonization Project, were presented at a public meeting on February 1st and focused on the homologous recombination deficiency (HRD) biomarker in ovarian cancer. The project generated data to answer the questions: Do HRD assays report different HRD results and, if so, why might this variation exist?
To answer these questions, Friends convened with stakeholders and provided test developers with two common sets of ovarian cancer samples – 1) an in silico (i.e., computer based) dataset and 2) a clinical dataset of freshly extracted tumor samples. Each test developer used their own approach to determine the HRD status of each sample. NCI statisticians assessed which differences in the testing characteristics and approaches contributed to observed variability in the results.
These data and analyses provide a better understanding of factors that drive variability in HRD test results and can inform recommendations for refining development to support alignment and ensure patients and providers receive accurate and reliable results regardless of the test used. The findings showed that there was moderate variability in assay outputs. Moving forward it was recommended that assay developers work with the community to identify the best approach for reporting HRD to enhance consistency, to align on expectations for analytical validation, and to consider approaches for developing a “gold standard,” including use of a reference material.
Important News in Cancer Research
- On February 1st, Friends launched the Digital and Computational Pathology Tool Harmonization (Digital PATH) Project to identify factors that may contribute to variability in biomarker assessment across computational pathology platforms, propose areas for alignment in the field, and provide insights for shaping regulatory processes. Read more here.
- FDA has long prioritized racial and ethnic diversity in research studies to ensure clinical trials are representative of the patients who will receive the product. In 2022, Congress passed legislation that provides FDA the authority to require that sponsors submit Diversity Action Plans (DAPS) for clinical trials supporting approval. Guidance on what should be included in DAPs was expected at the end of 2023 and has not yet been issued; however, the FDA remains committed to fulfilling this requirement. Efforts to engage diverse communities in clinical trial recruitment were discussed in a recent meeting hosted by Friends. Read more here.
- The FDA’s approval of the first cell therapy for solid tumors is a significant milestone in cancer treatment advancement, particularly for advanced melanoma patients, although concerns remain regarding accessibility, cost, and the rigorous treatment regimen. Read more here.
Did you know?
Diagnostic tests, particularly Laboratory Developed Tests (LDTs), have historically been regulated under the Clinical Laboratory Improvements Amendments (CLIA), which primarily evaluates lab performance rather than the accuracy of the tests themselves. While FDA has maintained it has the authority to regulate LDTs as medical devices, the agency has historically exercised enforcement discretion to not evaluate most LDTs. This past September, FDA released a proposed rule that makes explicit that LDTs are in vitro diagnostic products (IVDs), and thus, subject to FDA oversight. This proposed rule is the most recent development in a decade-long effort to modernize diagnostic oversight in which both FDA and Congress have attempted to revise the approach to regulating IVDs offered as LDTs. Friends harmonization projects provide insights into variability that can exist among diagnostics and offer viable solutions to enhance alignment and review. Friends submitted a public comment on the proposed rule and continues to support transparency in test performance and appropriate oversight of diagnostics.
Mark Your Calendars
If you’re interested in supporting an event hosted by Friends, please email advocacy@focr.org
^† April 2nd – High Fidelity: Advancing Use of Novel Technologies Through Collaboration
† April 3rd – 5th – Duke Industry Statistics Symposium (DISS 2024)
* April 4th – 5th – 2024 Community Oncology Conference
* April 7th – 10th – AACR Annual Meeting
^† May 6th – Cell Therapies Public Meeting; Co-hosted by Friends and the Parker Institute for Immunotherapy; stay tuned for details
May 15th – 16th – Food and Drug Law Institute Annual Conference
* June 2nd – 6th – ASCO Annual Meeting
* = Reduced Registration for Advocates
^ = Free to Attend
† = Friends Staff Presenting
In Case You Missed It (December 15th – March 15th)
- On February 1st, Friends hosted “The Future of Diagnostic Tests: New Data & Modern Policy.” Read the recap or rewatch the meeting here.
- On February 15th, Friends, Aetion, Duke-Margolis Center for Health Policy, and Reagan-Udall Foundation for the FDA co-hosted “Advancing Real-World Data to Generate Regulatory Grade Real-World Evidence in Oncology.” Rewatch the meeting here.
- On February 20th, Friends hosted “Enhancing Diversity in Clinical Trials: Implementation of Diversity Plans.” Read the recap or rewatch the meeting here.
- On February 26th, Friends and Prevision Policy co-hosted BioPharma Congress.
- Friends released its Annual Scientific Report for 2023. The report includes all publications released by Friends over the past year and highlights how our work continues to move the field forward.
ProgressforPatients.org
ProgressforPatients.org is an online advocacy education program created by Friends to help patients, advocates, and caregivers acquire the necessary tools to effectively communicate with drug researchers, developers, and regulators. If you’re interested, we encourage you to take our free advocacy education course found here.

