Physicians rely on a variety of sources of information to provide high-quality care to patients. In oncology, some of the most prominent resources that inform provider decision-making are the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) clinical practice guidelines; drug compendia; electronic point-of-care decision support tools, such as UpToDate; and Food and Drug Administration (FDA)-approved drug labeling.i ii iii iv v
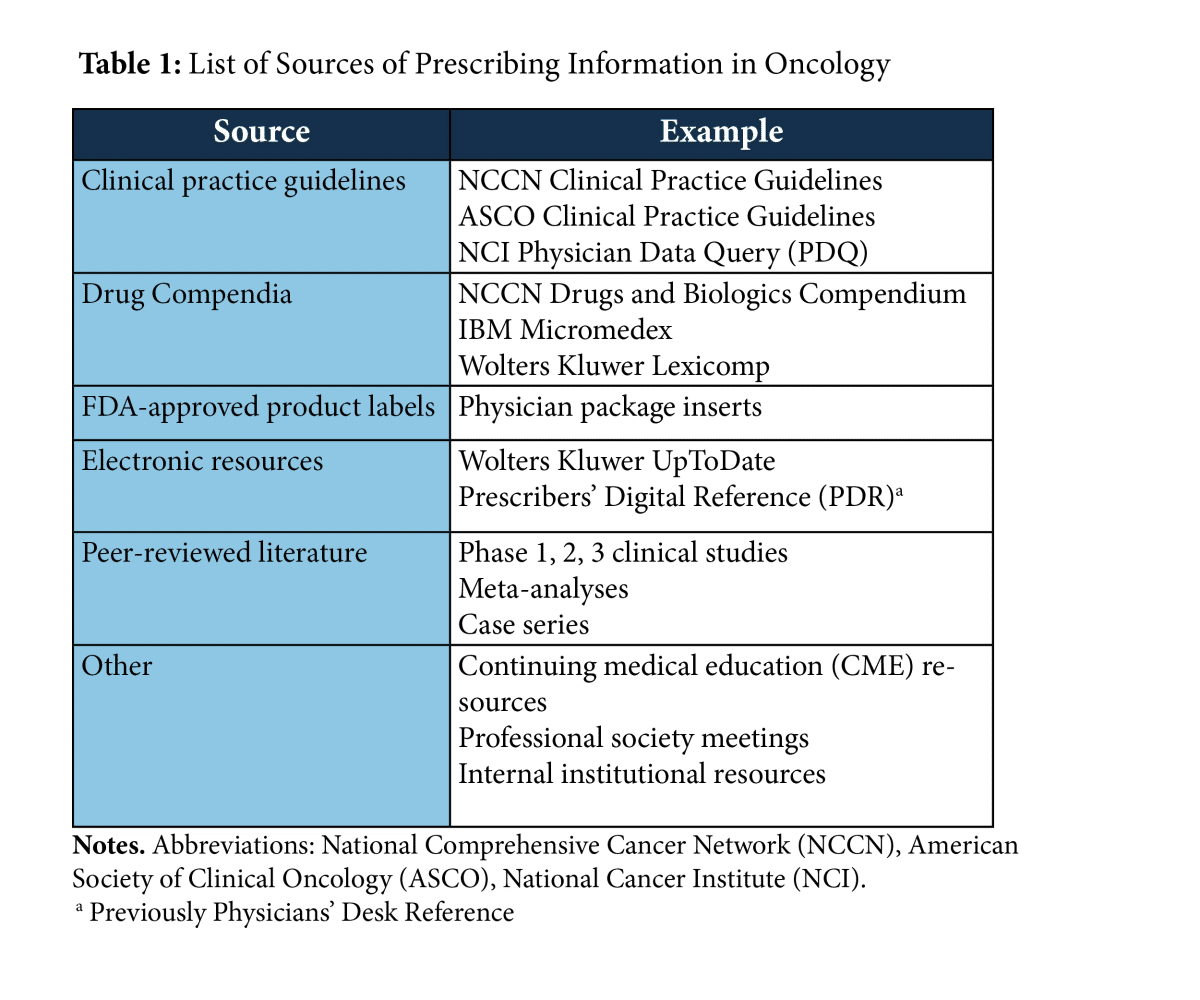
Each of these resources have benefits and drawbacks, which are due to the differences in the type of evidence each is supported by and the way that evidence is presented. As an example, drug labeling is carefully vetted by the FDA for accuracy and cannot contain false, misleading, or promotional information.vi Therefore, it may reasonably be relied on as a source of high quality scientific information. Unfortunately, labels can quickly fall out of date with important, new information relevant to safe and effective uses failing to be incorporated.vii In contrast, other resources may provide prescribers with more readily updated information but may fail to provide the same assurances of accuracy and reliability.
To understand how drug labeling is perceived in relation to the other major sources of prescribing information, we collaborated with the Deerfield Institute to develop a brief online physician questionnaire. The questionnaire sought to determine 1) how often different sources of prescribing information are relied upon for decision-making, and 2) how physicians rate different sources of prescribing information based on several metrics, including timeliness, scientific rigor, etc.
These questions help us better understand the prescribing resources most utilized by physicians and the metrics that physicians seek when relying on a specific source of prescribing information.
SURVEY QUESTIONS AND DATA
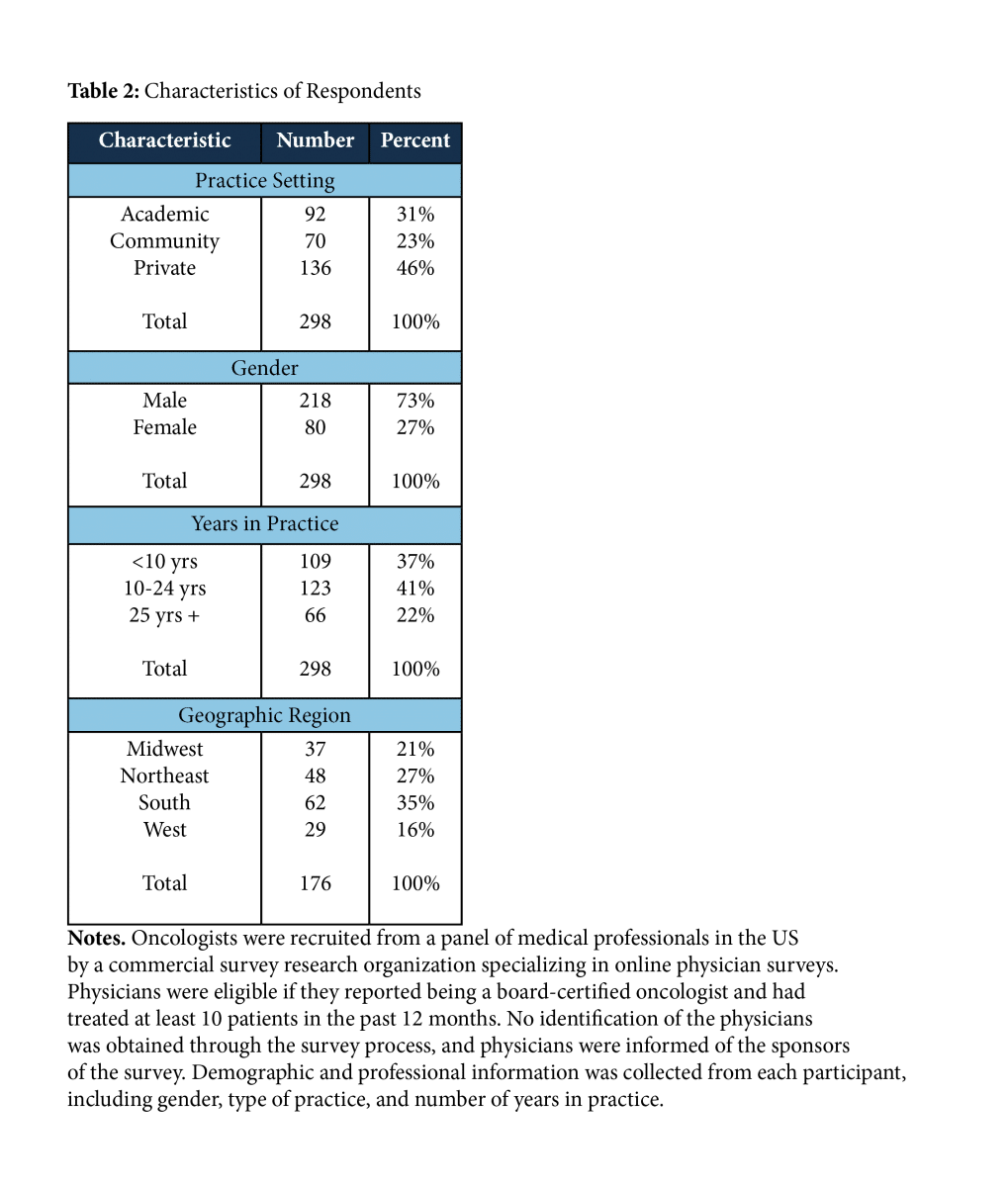
A total of 298 oncologists with the following characteristics completed the survey.
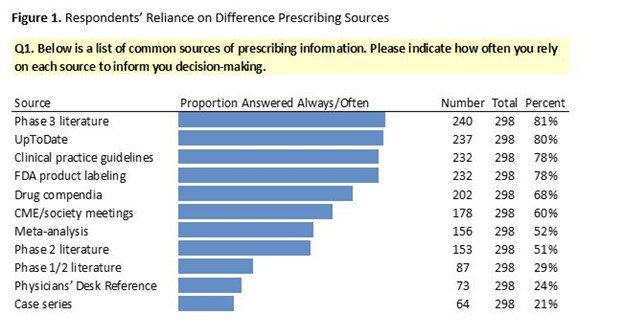
Notes. Item 1 of the questionnaire asked respondents to indicate which sources of prescribing information they rely on. Answer choices were “Always”, “Very often”, “Sometimes”, “Rarely”, “Never”, “Not applicable”. The above figure summarizes proportions of responses that were “Always” or “Very often”.
Respondents reported that they rely on multiple sources of information when making prescribing decisions. We found evidence that most oncologists rely upon the following six sources “Always” or “Often” when making prescribing decisions: phase 3 peer-reviewed literature, UpToDate, FDA product labeling, clinical practice guidelines, drug compendia, and CME/society meetings.
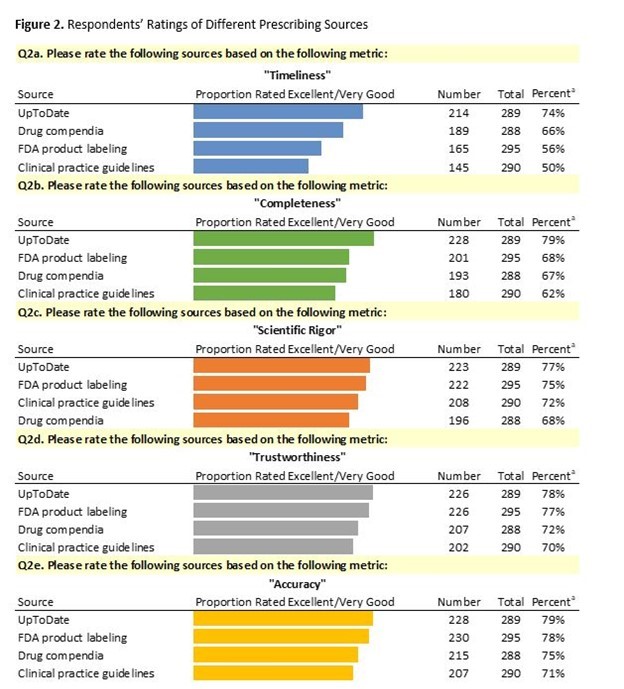
Notes. Item 2 of the questionnaire asked respondents to rate the following major sources of prescribing information: FDA-approved product labeling, clinical practice guidelines, UpToDate and drug compendia. Answer choices were “Excellent”, “Very good”, “Good”, “Satisfactory”, “Poor”, “Unsure”. The above figure summarizes proportions of responses that were “Excellent” or “Very good”.
Respondents gave positive ratings to each of the major sources of prescribing information. We found some significant differences based on “Completeness” and “Timeliness”. Differences were observed between UpToDate and FDA-approved product labels based on “Timeliness”. Differences were observed between UpToDate and clinical practice guidelines based on “Timeliness” and “Completeness”.
FINDINGS
The results indicate that oncologists have favorable perceptions of the major prescribing resources in their field and use multiple sources when making decisions. While each of the four major sources were rated highly on a series of quality metrics, UpToDate stood out as the source with the most consistently high ratings, suggesting that “Timeliness” is a particularly important consideration for prescribers.
A noteworthy finding from this survey is that respondents reported relying on FDA-approved product labels nearly as often as clinical guidelines and UpToDate. In the past, physicians have been found to have limited knowledge of FDA-approved indications of drugs vs. unapproved uses, suggesting low levels of use of labeling.viii Oncologists who completed this survey reported relying upon labels more than previously thought. Policymakers should consider ways to ensure drug labeling continue to contain high-quality evidence that contains timely information.
As with any web-based survey, this study is susceptible to both selection and reporting biases. Although we believe the data provide important directional information on how oncologists perceive the sources of information investigated in this survey, caution should be used when generalizing results of this subset of physicians to the entire oncologist population.
Acknowledgements
We thank William Wood (University of North Carolina, Chapel Hill, NC) for reviewing the data and providing helpful comments. We also thank Hugh Van Dyke (American University, Washington, DC) for statistical analysis support. Financial support for this study was provided by Deerfield Management, a healthcare investment firm dedicated to advancing healthcare through investment, information, and philanthropy.
References
i Guidelines, Tools, & Resources. c2018. Alexandria (VA). American Society of Clinical Oncology; [accessed August 2018]. https://www.asco.org/practice-guidelines/quality-guidelines/guidelines
ii NCCN Guidelines & Clinical Resources. c2018. Fort Washington (PA). National Comprehensive Cancer Network; [accessed August 2018]. https://www.nccn.org/professionals/physician_gls/default.aspx
iii Lexicomp Online. c2018. Indianapolis (IN). Wolters Kluwer; [accessed August 2018]. http://www.wolterskluwercdi.com/lexicomp-online/
iv UpToDate. c2018. Indianapolis (IN). Wolters Kluwer; [accessed August 2018]. https://www.uptodate.com/home
v Drugs@FDA: FDA Approved Drug Products. Silver Spring (MD). US Food and Drug Administration; [accessed August 2018]. https://www.accessdata.fda.gov/scripts/cder/daf/
vi 21 Code of Federal Regulations 201.56.
vii Shea MB, Stewart M, Van Dyke H et al. Outdated Prescription Drug Labeling: How FDA-Approved Prescribing Information Lags Behind Real-World Clinical Practice. THER INNOV REGUL SCI. 2018 [Epub ahead of print].
viii Chen DT, Wynia MK, Moloney RM, Alexander GC. U.S. physician knowledge of the FDA-approved indications and evidence base for commonly prescribed drugs: results of a national survey. PHARMACOEPIDEMIOL DRUG SAF. 2009;18(11):1094-1100.