Washington, DC – The Artificial Intelligence-Based Measurement of Response Evaluation Criteria in Solid Tumors (ai.RECIST) Project aims to answer the question: Can artificial intelligence (AI)-based imaging tools improve tumor measurement? By enhancing the speed and reliability of tumor measurements, AI has the potential to accelerate clinical trial progress and improve patient access to effective treatments.
Visit the ai.RECIST Project Page
In clinical trials, radiologists at local trial sites measure tumors, with these measurements later verified through blinded independent central review (BICR). While this approach helps ensure consistency and reduce bias, it also increases the time and resources necessary to complete clinical studies. The validation and use of AI tools could help streamline assessment.
Tumor measurements follow standardized, Response Evaluation Criteria in Solid Tumors (RECIST) criteria applied by expert human readers. While RECIST provides a systematic framework for objective tumor measurements, implementation faces several challenges, including investigator bias, subjectivity in lesion selection, and variability in measurements across clinical sites and radiologists.
“AI-driven tumor measurement tools have the potential to add considerable efficiency to clinical trials. This new project will provide a greater understanding of tool performance and help establish validation approaches to support their use in future studies.” Friends of Cancer Research President and CEO Jeff Allen.
Initial project objectives will focus on assessing AI tool agreement, comparing variability among AI tools and human readers, and using these findings to explore opportunities for leveraging AI tools in clinical trials and proposing best practices for implementation. To support these objectives, the project will use a common dataset from lung cancer trials, providing a consistent foundation to evaluate tool performance and variability in a relevant clinical setting.
ai.RECIST Project Phases
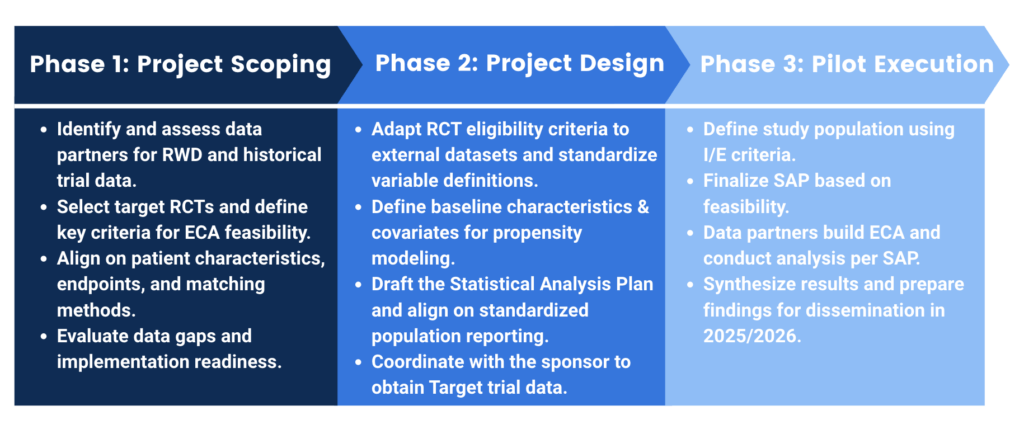
This project is made possible through partnerships across academia, government, industry, and patient advocates, who recognize the critical need to streamline and enhance the accuracy of tumor measurement in clinical trials.
Project Partners
Amgen, Atlis Labs, AstraZeneca, Foundation for the National Institutes of Health, Friends Advisory Advocates, Genmab, GSK, Johnson and Johnson Innovative Medicine, Eli Lilly and Company, LivAI, Lunit, MD Anderson Cancer Center, Merck and Co., Inc., Memorial Sloan Kettering Cancer Center, Novartis, Pfizer, Picture Health, University of Pittsburgh Medical Center, Project Data Sphere®, Quibim, Qure AI, Radiomics.bio, Takeda Pharmaceuticals, Tempus AI, Temple University, Voiant, and Vysioneer.
This project builds on Friends Digital PATH Project, which examined variability and potential sources of discordance across models assessing HER2 status in breast cancer. The findings from that project helped inform alignment efforts and proposed solutions to improve model development and validation.
To learn more about the project visit: https://friendsofcancerresearch.org/ai-recist/

