A new publication by Friends of Cancer Research (Friends) was published in Clinical Cancer Research, a journal of the American Association for Cancer Research, “An evaluation of novel oncology approvals with a PMR/C for assessing data in racial and ethnic populations underrepresented in premarket clinical trials.” This important publication used publicly available data to characterize postmarketing requirements and commitments (PMR/Cs) over the last decade, providing key insights into the FDA’s expectations for representation of race and ethnicity in oncology clinical trials.
This manuscript is the second in a series of Friends’ publications evaluating PMR/Cs issued over the last decade.
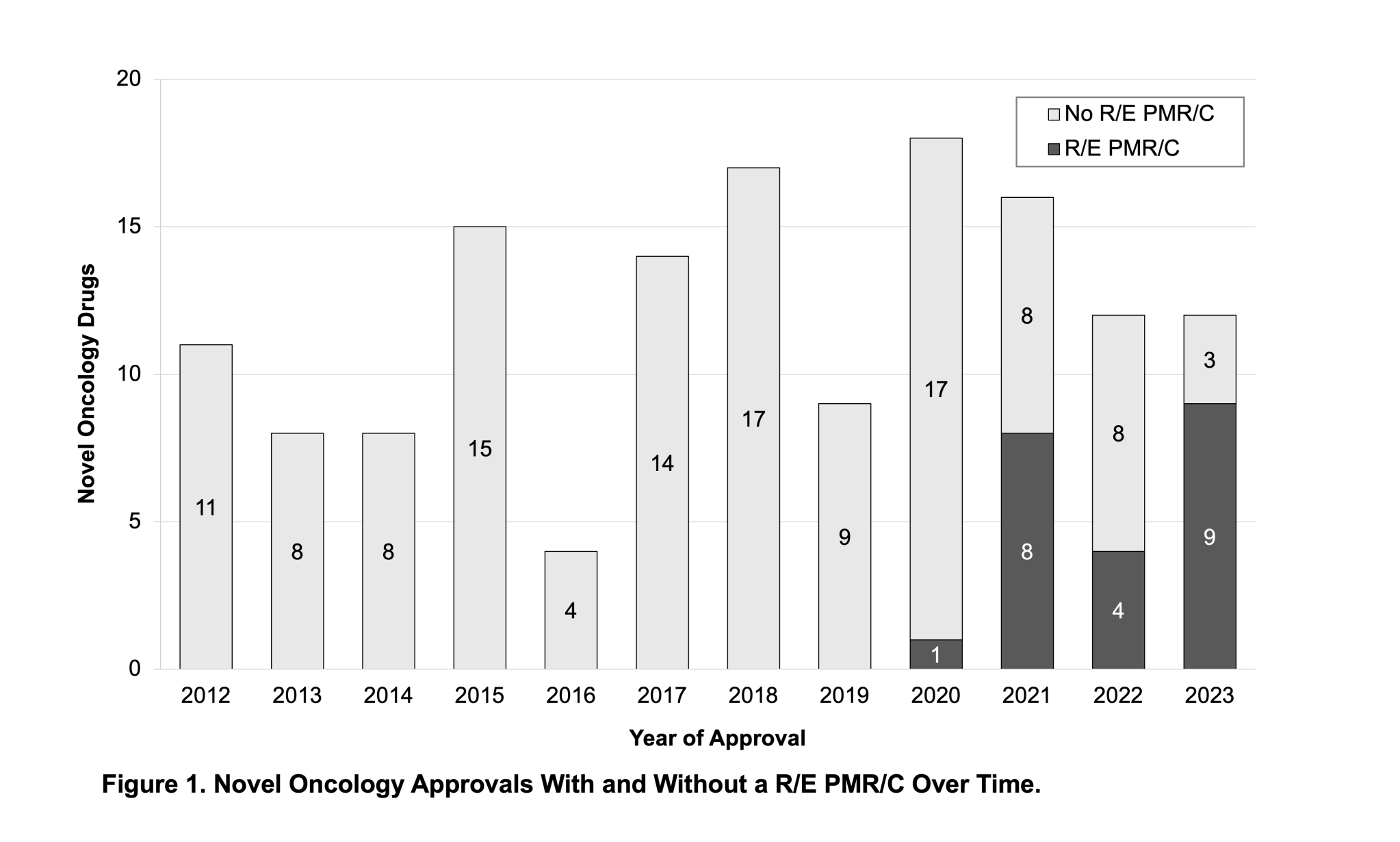
“This research shows that prior to 2020, no approved drugs had race or ethnicity related PMR/C, while in the last three years, more than half of novel oncology approvals have had such a PMR/C (21/40, 53%),” said Dr. Jeff Allen, President & CEO of Friends. “We hope the results from this study help demonstrate the importance for collecting these data and can inform future trial designs to better characterize use in a more representative population,” Dr. Allen added.
“Additionally, we observed that certain approval characteristics may contribute to decisions to issue a PMR/C to conduct a study that is more representative of the racial and ethnic diversity of the U.S. or intended patient population,” said Grace Collins, Regulatory Policy Analyst at Friends.
This publication provides important insights about strategies for ensuring clinical trials are more inclusive and representative of the intended patient population and general U.S. population.
Friends established a PMR/Cs dashboard using data from publicly available approval letters and review documents in FDA’s database, serving as the basis for the data analyzed in this publication. The dashboard displays data on PMR/Cs issued at the time of initial approval for novel oncology therapies between January 1, 2012, and December 31, 2023.
Learn more about how to use this resource and access the dashboard here.
Read our previous PMR/C paper published in Clinical Cancer Research focused on dosing-related postmarketing requirements, here.
Manuscript Authors
Jeff D. Allen, Hillary S. Andrews, Grace Collins, Brittany McKelvey, Carrigan Rice, Mark D. Stewart.
About Friends of Cancer Research
Friends of Cancer Research (Friends) is working to accelerate policy change, support groundbreaking science, and deliver new therapies to patients quickly and safely. We unite scientists, industry researchers, patient advocates, and policy makers to build unique collaborations able to instigate progress faster than any one organization working alone. This collaboration among partners from every healthcare sector ultimately drives advances in science, policy, and regulation that speed life-saving treatments to patients.
For more information, please visit https://friendsofcancerresearch.org/.