Washington, DC – May 7, 2024 – A new publication by Friends of Cancer Research (Friends) was recently published in the journal Diagnostics: “Advancing Evidence Generation for ctDNA: Lessons Learned from A Multi-Assay Study of Baseline ctDNA Levels Across Cancer Types and Stages.” This collaborative publication evaluates circulating tumor DNA (ctDNA) levels prior to cancer treatment (“baseline”) across eight commercial ctDNA assays in five different cancer types to identify overall trends and considerations to inform the use of ctDNA in oncology drug development.
“This unique partnership provides foundational evidence regarding ctDNA levels across different tumor types and stages and identifies opportunities for future alignment of ctDNA measurement.” said Dr. Jeff Allen, President & CEO of Friends.
“To support the future use of ctDNA as an early endpoint, meta-analyses are needed for multiple cancer types and stages, and likely will include data from multiple ctDNA assays,” said Dr. Brittany Avin McKelvey, Director of Regulatory Affairs at Friends. Our work helps support these necessary analyses.
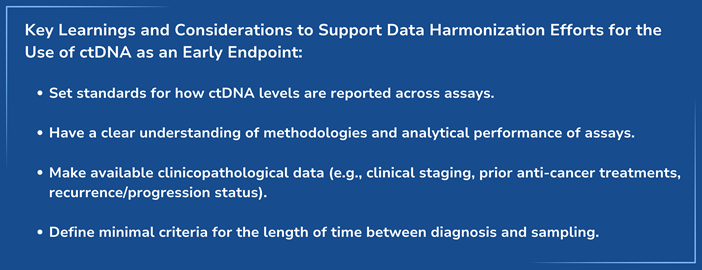
This work emerged from Friends’ 2022 Evidentiary Roadmap for generating evidence to support the use of ctDNA as an early endpoint, which proposed identifying clinical and technological characteristics that influence monitoring ctDNA levels across disease settings to support a rationale for evidence generation across cancer types. The manuscript describes baseline ctDNA levels across cancer types and assays and underscores the importance of evaluating factors such as assay analytical performance and methodology, and the development of common data standards and key performance characteristics to generate robust data.
To learn more about Friends latest ctDNA work and the initiatives that led to our current findings please visit our ctMoniTR Project here.
Manuscript Authors
Brittany A. McKelvey, Hillary S. Andrews, Frederick L. Baehner, James Chen, Carin R. Espenschied, David Fabrizio, Vanessa Gorton, Claire Gould, Justin Guinney, Greg Jones, Xiangyang Lv, Michael S. Nahorski, Melanie R. Palomares, Gary A. Pestano, Mark Sausen, Alain Silk, Nicole Zhang, Zhihong Zhang, Mark D. Stewart, and Jeff D. Allen.
Manuscript Partners
Biodesix Inc., Burning Rock, Exact Sciences Corp., Foundation Medicine, Inc., Guardant Health, Inc., NeoGenomics Laboratories, Personal Genome Diagnostics, Predicine, Tempus AI, Inc.
About Friends of Cancer Research
Friends of Cancer Research (Friends) is working to accelerate policy change, support groundbreaking science, and deliver new therapies to patients quickly and safely. We unite scientists, industry researchers, patient advocates, and policy makers to build unique collaborations able to instigate progress faster than any one organization working alone. This collaboration among partners from every healthcare sector ultimately drives advances in science, policy, and regulation that speed life-saving treatments to patients.
For more information, please visit https://friendsofcancerresearch.org/.

