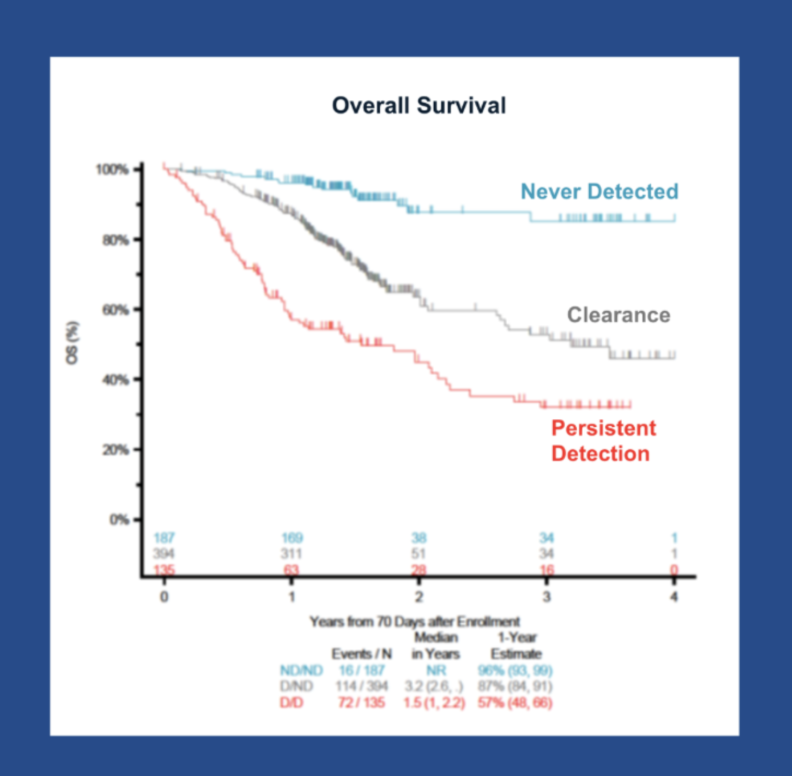
Washington, DC – New findings from Friends of Cancer Research (Friends)’s ctMoniTR Project were published in Clinical Cancer Research, a journal of the American Association for Cancer Research. This important publication demonstrated in an aggregate analysis of eight clinical trials of patients with advanced non-small cell lung cancer (aNSCLC) treated with tyrosine kinase inhibitors (TKI), circulating tumor DNA (ctDNA) clearance associates with improved long-term outcomes, including overall survival (OS). Additional analyses assessed the relationship between ctDNA change and Early Response Evaluation Criteria In Solid Tumors (RECIST) measurements with long-term outcomes including OS and progression-free survival.
“Interestingly, we saw clearance of ctDNA associates with OS not only in the overall dataset, but also among patients with stable disease measured at their first RECIST timepoint, suggesting that outcomes for patients with early stable disease who often have heterogeneous survival might be better understood with earlier ctDNA data,” said Dr. Antje Hoering, President and CEO of Cancer Research And Biostatistics (CRAB), the publication’s co-author and ctMoniTR Project partner.
“This study builds on Friends’ led work, focusing on whether change in ctDNA levels could serve as an intermediate endpoint in drug development” said Dr. Jeff Allen, President and CEO of Friends. “These findings show that ctDNA clearance, rather than just a partial decrease as observed in aNSCLC treated with immunotherapy, associates with improved OS.”
The ctMoniTR Project is currently assessing change in ctDNA levels and associations with OS in a new set of aggregate clinical trials of patients with aNSCLC treated with immunotherapy (with or without chemotherapy) or chemotherapy alone to understand change in ctDNA in different treatment modalities.
Click to read the full publication
To learn more about Friends’ ctMoniTR portfolio and the projects, events and findings that have led to this important publication, click here.
A special thank you to the ctMoniTR Statistical Working Group members and the Broad Working Group members for making this research partnership and publication possible.
Manuscript Authors
Hillary S. Andrews, Nevine Zariffa, Katherine K. Nishimura, Stephanie H. Choi, Shibing Deng, Megan Eisele, Carin R. Espenschied, Emily M. Goren, Minakshi Guha, Samuel Hong, Dilafruz Juraeva, Nicole Krämer, Li Liu, Jean-Francois Martini, Brittany A. McKelvey, Geoffrey R. Oxnard, Gary A. Pestano, Lynne Poole, Adam Rosenthal, Anna M. Szpurka, Diana Merino Vega, Christine Ward, Sameera R. Wijayawardana, Antje Hoering, Mark D. Stewart, Jeff D. Allen.
Manuscript Partners
Agilent Technologies, AstraZeneca, Biodesix Inc., Boehringer Ingelheim Pharma GmbH & Co KG., Cancer Research And Biostatistics, Eli Lilly and Company, EMD Serono, Friends of Cancer Research (Friends), Guardant Health, Inc., Illumina, Inc., Loxo@Lilly, The healthcare business of Merck KGaA, Darmstadt, Germany, NMD Group Inc., Pfizer, Takeda Pharmaceuticals Inc., U.S. Food and Drug Administration.

