Findings by Friends of Cancer Research (Friends) and collaborators were published today in JCO Oncology Advances, “An Analysis of 20 Independently Performed Assays to Measure Homologous Recombination Deficiency (HRD) in Ovarian Cancer: Findings from the Friends’ HRD Harmonization Project.”
This manuscript examines variability in outputs from HRD assays assessing a common sample set. HRD is a biomarker used to determine eligibility for Poly ADP-ribose Polymerase Inhibitors (PARPi) to treat patients with high-grade serous ovarian carcinoma (HGSOC). The findings demonstrate variability in HRD results across assays, suggesting the need for consistent and aligned strategies in biomarker selection for clinical trials and clinical decision-making in oncology drug development.
“HRD assays measure DNA damage repair dysfunction to identify patients with ovarian cancer who could potentially benefit from drugs like PARPi,” stated Dr. Ethan Sokol, Principal Scientist, Foundation Medicine. “It is important that all HRD assays provide optimal clinical performance for predicting treatment response.”
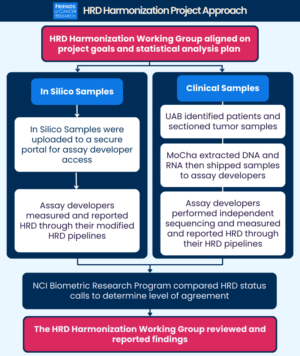
These findings build on Friends Diagnostics Harmonization Portfolio, which aims to assess variability across different diagnostic tests and inform regulatory frameworks to improve test performance. This body of work emphasizes the need for improved standardization and alignment in assay reporting. By identifying sources of assay discordance, this research paves the way for improvements in assay consistency and reliability, which are essential for optimizing clinical decisions and effectiveness of therapeutic strategies in oncology drug development.
“While the analysis set the groundwork for what needs to occur to create alignment, we did not focus on a gold standard due to a lack of agreement on what that might be,” said Dr. Lisa McShane Associate Director, Division of Cancer Treatment and Diagnosis at the National Cancer Institute. “For all diagnostic development, it is important that gold standards and reference datasets are established to improve assay output consistency.”
Ongoing work from the Friends Diagnostics Harmonization Portfolio will be discussed at our upcoming February 4th diagnostics event where we will explore innovative strategies, regulatory flexibility, and the validation for reliable diagnostics in oncology. Friends thanks the co-authors and collaborators for their time and support!
Read the full publication here
click here to learn how Friends HRD Harmonization Project supports assay consistency and clinical decision-making by addressing variability in HRD testing.
Manuscript Authors
Hillary S. Andrews, Lisa M. McShane, Elise C. Kohn, Rebecca Arend, Chris Karlovich, Kaitlyn Kincaid, A. Douglas Laird, Ming-Chung Li, Ethan S. Sokol, Elizabeth R. Starks, Shulin Bi, Lauren Brunner, Alyssa Chapman, Li Chen, Tommaso Coletta, Yuan Ding, Bailee Dover, McKenzie Foxall, Mohit Gupta, Zan Halford, Andrea G. Kahn, Nikita Kotlov, Yi-Hsuan Lucy Lai, Alexander J. Lazar, Wenjie Li, Brittany A. McKelvey, Hyunjun Nam, Sarabjot Pabla, Pegah Safabakhsh, Daniel Saul, Albrecht Stenzinger, Timothy Taxter, Zhiwei Zhang, Yingdong Zhao, ShiPing Zou, Mark D. Stewart, Jeff D. Allen.
Manuscript Partners
Friends of Cancer Research, National Cancer Institute, University of Alabama Birmingham, Frederick National Laboratory for Cancer Research, Pfizer, Inc., Foundation Medicine, Invitae Corporation, Amoy Diagnostics Co., Ltd., PathAI, SOPHiA GENETICS, Illumina, Inc., Thermo Fisher Scientific, Diagnostic Laboratory Services, Inc., BostonGene, ACT Genomics Co., LTD., The University of Texas MD Anderson Cancer Center, Burning Rock Dx, NeoGenomics Laboratories, Labcorp Oncology (OmniSeq), Guardant Health, Inc., Bionano Genomics, Inc., University Hospital Heidelberg, Tempus AI, Pillar Biosciences.

