A new analysis from Friends of Cancer Research (Friends) characterizes flexibilities utilized in the development of companion diagnostics (CDx). Reviewing Summary of Safety and Effectiveness Data (SSED) documents from FDA Premarket Approval applications (PMA) for non-small cell lung cancer (NSCLC), the analysis describes how alternative sample sources can be leveraged for CDx validation, particularly when sample availability is limited for rare cancer biomarkers.
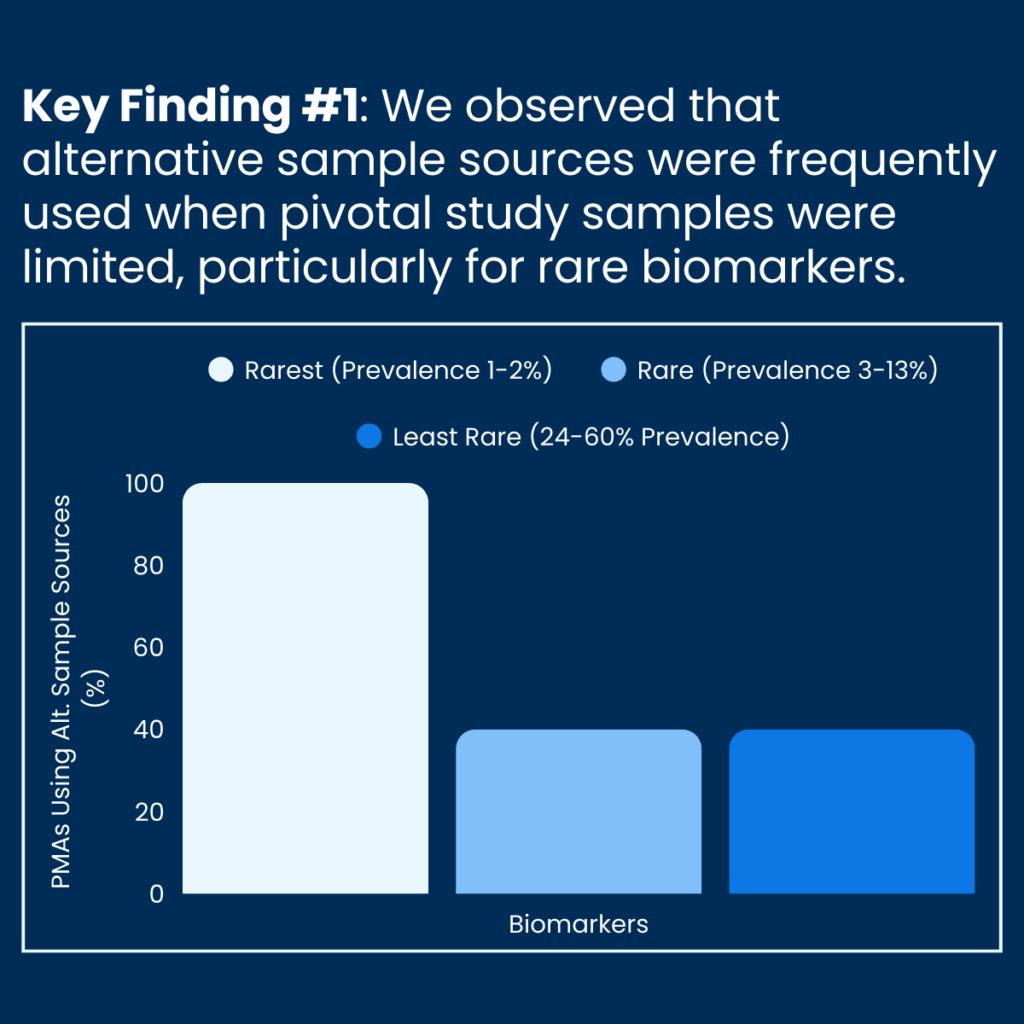
As precision medicine advances, reliable diagnostic tests are critical for identifying patients most likely to benefit from targeted therapies. Yet when biomarkers are rare—particularly those occurring in less than 1% of patients with a given indication—developers often face challenges in acquiring sufficient pivotal clinical trial specimens to support standard validation approaches, potentially delaying both approval and timely patient access to novel treatments.
Additionally, the analysis revealed that bridging studies for the rarest biomarkers contained fewer samples compared to those for more common biomarkers.
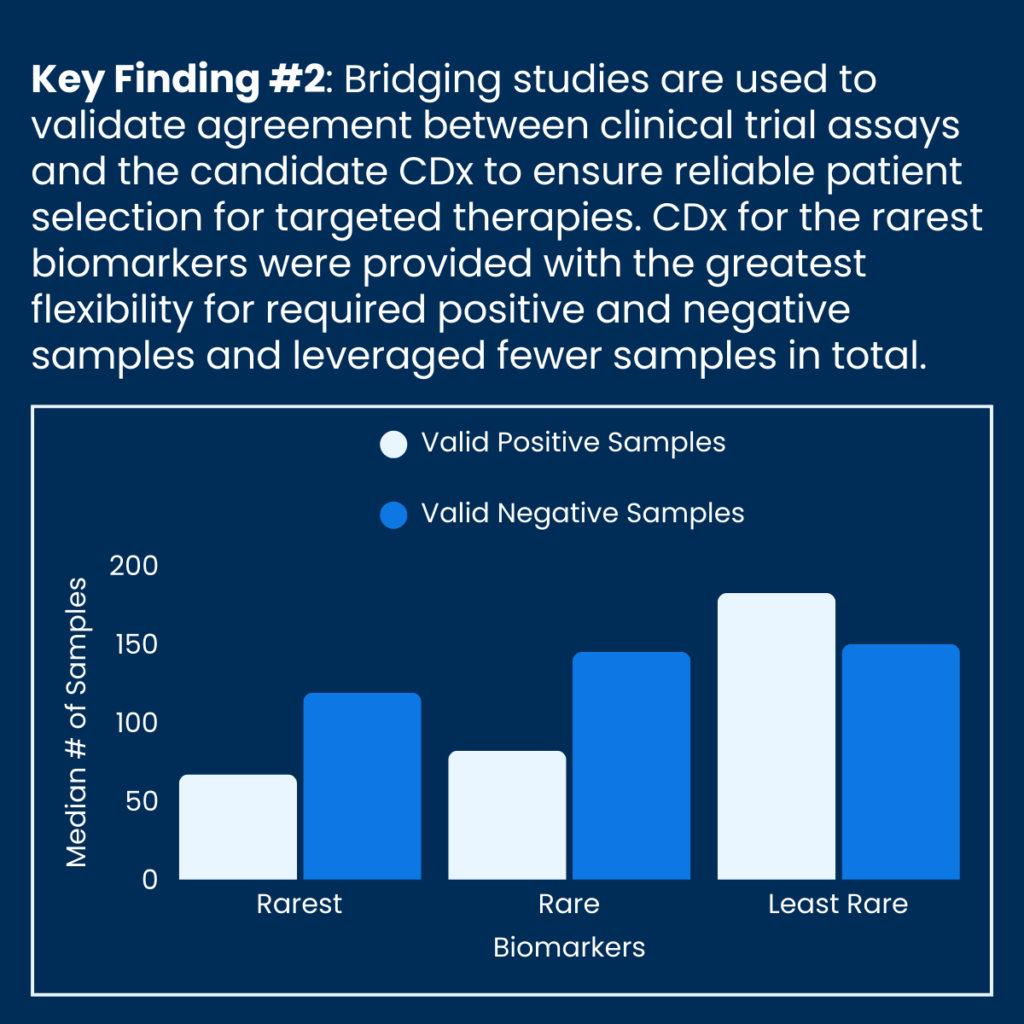
The study authors highlight how regulatory flexibilities can be appropriate in situations where sample availability is limited. The development of regulatory guidance regarding the appropriate use of alternative samples would streamline development and help ensure timely patient access to potentially life-saving precision medicine therapies.
Companion Diagnostic FDA Review Flexibilities: An Assessment of CDx for NSCLC to Support Aligned Approaches for Validation is published in Therapeutic Innovation & Regulatory Science (TIRS), a scientific journal of the Drug Information Association.
Read it here: https://link.springer.com/article/10.1007/s43441-025-00799-7.

