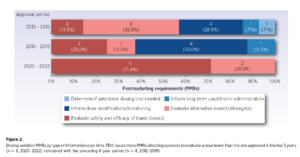
A new manuscript by Friends of Cancer Research (Friends) published today in AACR’s Clinical Cancer Research, shows that a substantial number of oncology drugs undergo additional dose evaluation after approval through Postmarketing Requirements (PMRs), studies that sponsors are required to conduct after approval to gather additional information about a product’s safety and/or efficacy.
These findings align with recent initiatives and FDA guidance that outline current thinking around the need for improved dose optimization in the pre-market setting. They also build on Friends’ work focused on Tolerability and Dosing.
“This analysis provides timely insights into the dosing activities that the FDA has requested in the postmarketing setting over the last decade,” said Dr. Jeff Allen, President & CEO of Friends of Cancer Research. “These findings reemphasize the importance of dose optimization strategies to maximize tolerability and favorable outcomes for patients.”
This information serves as a valuable guide to identify areas where more robust pre-market dose optimization studies may be needed to ensure the most optimal dose is identified prior to approval to help avoid lengthy post market studies and unnecessary patient exposure to a suboptimal dose.
A new interactive Friends data dashboard that contains data on all PMRs and Postmarketing Commitments (PMCs) issued for novel oncology drugs approved in the last ten years helped inform this analysis. The dashboard can be used to explore data on PMR/Cs including trends over time, by drug class, and types of data collected in the postmarket setting to glean additional insights.
Click to read the full manuscript
Manuscript Authors
Grace Collins, Brittany McKelvey, Hillary Andrews, Jeff D. Allen, Mark D. Stewart.
About Friends of Cancer Research
Friends of Cancer Research (Friends) is working to accelerate policy change, support groundbreaking science, and deliver new therapies to patients quickly and safely. We unite scientists, industry researchers, patient advocates, and policy makers to build unique collaborations able to instigate progress faster than any one organization working alone. This collaboration among partners from every healthcare sector ultimately drives advances in science, policy, and regulation that speed life-saving treatments to patients.

