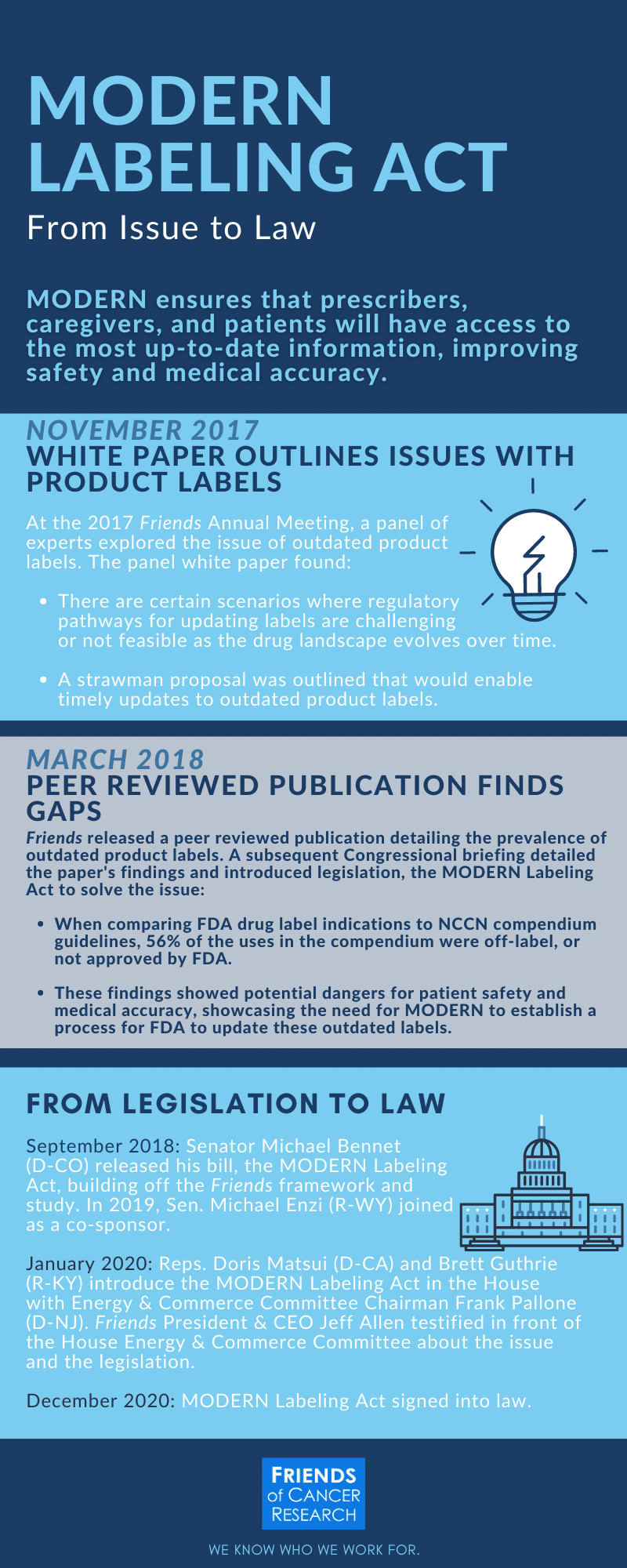
An Approach to Modernize Drug Labels
Federal regulations require that the labeling must contain a summary of scientific information about a drug, and that the information is informative and accurate. The content of the label is written by the original drug manufacturer and approved by the Food and Drug Administration (FDA) to ensure that it meets standards laid out in regulations.When older drugs are replaced by generics this can cause certain drug labels to become stuck in time.
The study identified more than four times as many uses for drugs on the National Comprehensive Cancer Network (NCCN) Drugs and Biologics Compendium than on drug labels (99 uses on labels vs. 451 uses in the Compendium), a staggering difference between two sources that both intended to offer prescribers guidance on the appropriate uses of cancer drugs. The report also found that 56% of the uses included in the Compendium were “off label,” meaning that these uses were not included in the FDA approved label. However, the FDA can only approve additional uses to labels if the manufacturer submits new data.
While many sources of information exist, none can deliver as strong assurances of reliability and scientific accuracy as FDA-approved product labels. Labels are the most carefully-vetted sources of prescribing information available today and play a critical role in safeguarding the public health.
Resources:
- Click HERE to access the Friends press release applauding the passage of the MODERN Labeling Act.
- Click HERE to access the Friends peer reviewed publication, “Outdated Prescription Drug Labeling: How FDA-Approved Prescribing Information Lags Behind Real-World Clinical Practice.”
- Click HERE to access the press release from the Senate HELP committee announcing the introduction of the S. 1895.
- Click HERE to access the press release from Sen. Bennet announcing the introduction of the bill to the Senate floor.
- Click HERE for a Friends’ announcement on the introduction of the bill to the Senate floor.
- Click HERE to read Congressional testimony from Friends President and CEO Jeff Allen on the MODERN Labeling Act.
- Click HERE for a post-event recap for the March Congressional briefing.
- Click HERE to access the Friends Annual Meeting whitepaper and click HERE for the panel video detailing the initial framework.

