2024 Impact Report: Powering Advances
5,000+
meeting attendees
15,000+
publication downloads
500+
working group participants
13
scientific & policy outputs
512
citations of our publications
10
conferences & roundtables
5,000+
meeting attendees
15,000+
publication downloads
500+
working group participants
13
scientific & policy outputs
512
citations of our publications
10
conferences & roundtables
Friends of Cancer Research (Friends) accelerates cutting edge cancer care that both extends and improves quality of life for patients. To accomplish this, we leverage groundbreaking collaborations, generate scientific evidence, and integrate patient input to shape public policy. This past year, our work was cited over 500 times, with 15,000 publication downloads and over 5,000 meeting attendees.
How we work at Friends:
- We identify problems standing in the way of innovative science reaching patients.
- We generate the necessary evidence to help overcome the problem.
- We create policy solutions based on this evidence.
Below is a snapshot of some of our research and policy programs with public readouts in 2024. We look forward to working with you in 2025.
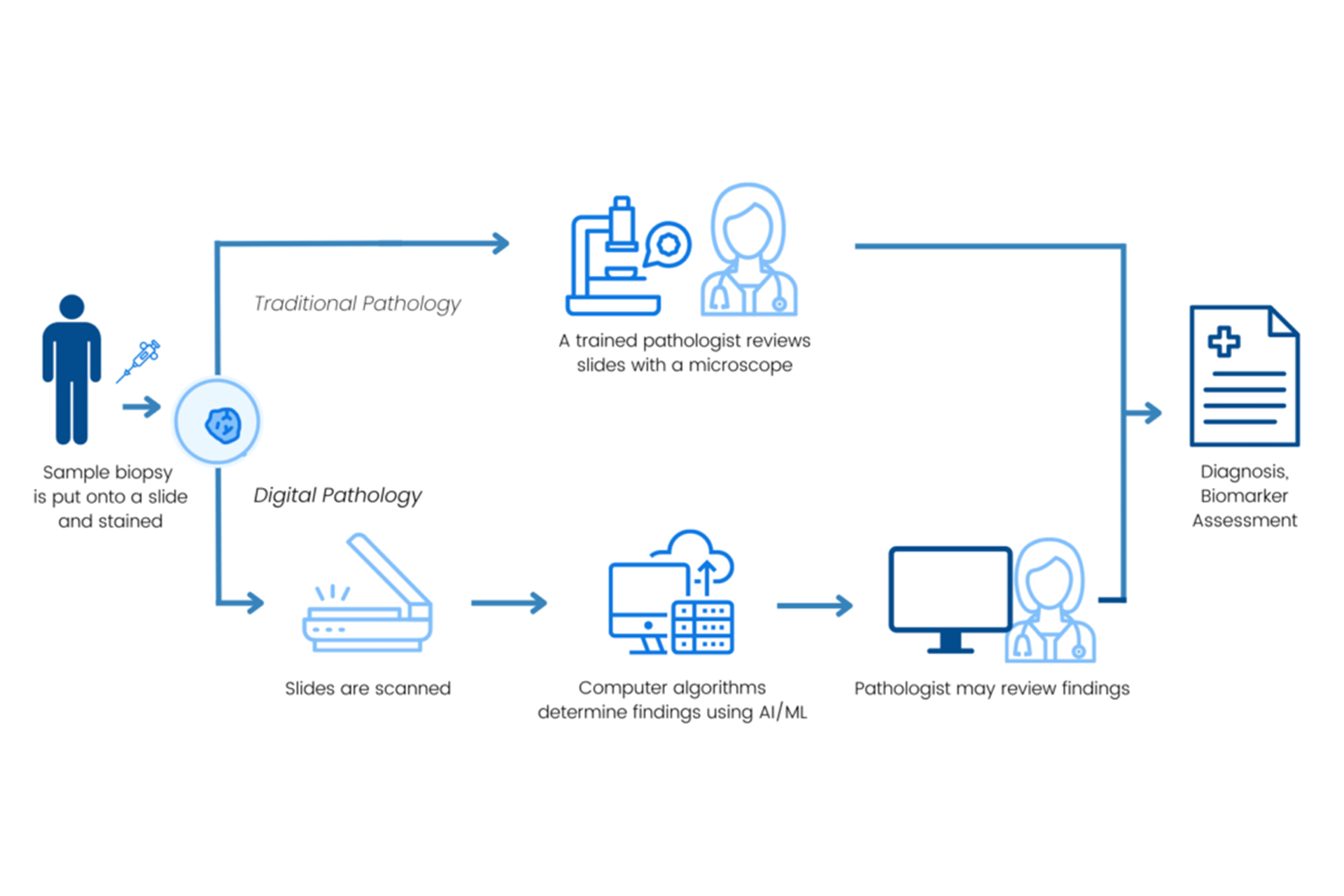
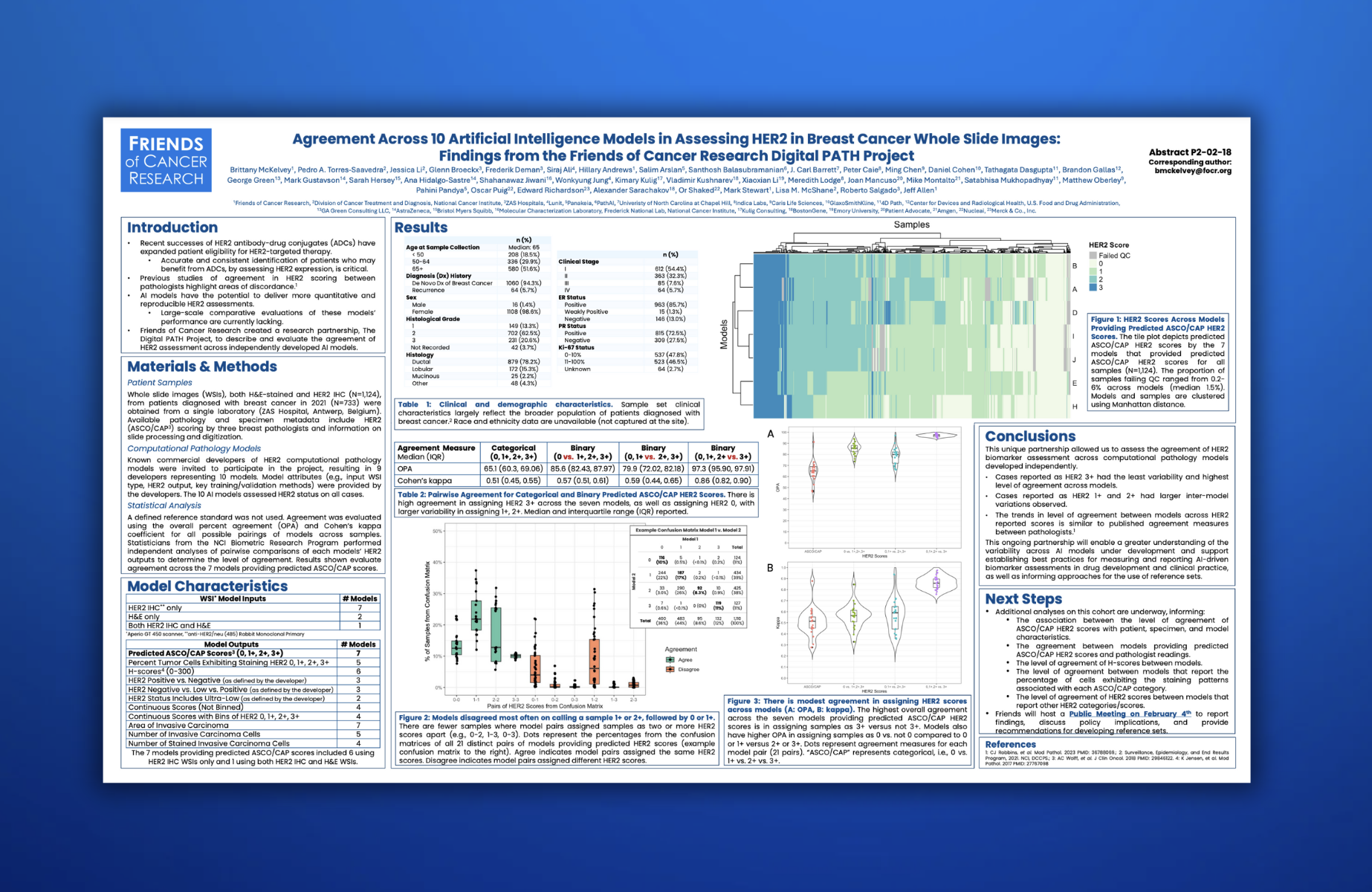
Digital PATH: Driving Innovation in Digital Pathology for Improved Cancer Diagnosis
Enhancing Biomarker Assessment Through Collaboration and Cutting-Edge Technology
We spearheaded efforts to enhance the accuracy and consistency of biomarker assessments across digital and computational pathology platforms. Digital pathology enables slide digitization for storage, viewing and analysis, sometimes incorporating computational pathology platforms that apply AI and machine learning algorithms to aid tissue evaluation. However, variability in outputs from these platforms presents a challenge. To address this, Friends convened a multi-disciplinary working group — including AI developers, patient advocates, regulators, pathologists, and drug developers— to assess HER2 biomarker measurement in breast cancer across computational pathology platforms.
Insights from the Friends’ Digital PATH Project contribute to broader discussions on how AI-enabled pathology tools may be integrated into regulatory frameworks, ultimately helping to establish reference data sets for model performance. By addressing variability and enhancing the accuracy and reproducibility of biomarker assessments, we aim to balance safety and innovation, ensuring that advancements in AI-enabled pathology tools lead to more reliable diagnoses, improved treatment selection, and ultimately, better outcomes for patients.
In 2025, Friends will launch the ai.RECIST Project, building on lessons learned from studying AI-tools in the Digital PATH Project, to evaluate AI-based imaging tools that assess tumor burden measurement. The first phase of the ai.RECIST Project will focus on assessing RECIST-based measurements to set the stage for exploring the full potential of AI-based imaging tools in measuring tumor burden.
HRD Harmonization Portfolio: Assessing variability to improve test performance
Standardizing and aligning assay reporting through diagnostic harmonization
Friends partnered with key stakeholders to assess sources of variability across HRD assays to identify areas for alignment and propose solutions to improve assay development. Our findings emphasize the need for improved standardization and alignment in assay reporting. By identifying sources of assay discordance, this project paves the way for improvements in their consistency and reliability.
This work is essential for improving clinical decisions and effectiveness of therapeutic strategies in oncology drug development, supporting consistency in results for patients and providers regardless of which assay is used to measure HRD.
- Friends Published a Manuscript in JCO OA Demonstrating Variability in HRD Results Across Assays, Suggesting the Need for Consistent and Aligned Strategies in Biomarker Selection.
- Friends’ Public Meeting Announced the HRD Harmonization Project Findings.
In 2025, Friends will use our HRD Harmonization Project findings to inform discussions on biomarker standardization and validation, supporting assay consistency and alignment in future diagnostic development.
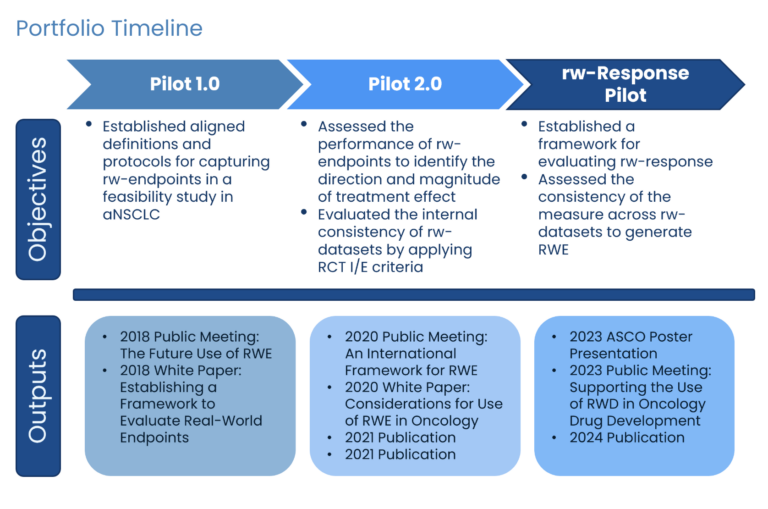
rw-Response Project: Promoting the Use of RWD in Oncology Drug Development
Establishing a Framework for Evaluating rw-Response
We are leading crucial work to advance methodology for using Real-World Evidence (RWD) as a more effective research tool. Our Real-World Evidence Portfolio established methodology for using RWD to evaluate treatment efficacy and safety for patients. Findings published in 2024 demonstrated the feasibility of aligning disparate real-world (rw) data sources to evaluate rw-response to treatment using clinician-documented response in patients with metastatic non-small cell lung cancer (mNSCLC).
Continued alignment of methodologies for aggregating and analyzing RWD will support use as a reliable and consistent source to generate RWE, support drug development, and regulatory decision-making.
In 2025, Friends will use the rw–Response Project to inform broader RWE initiatives, guiding best practices for evaluating real-world response data and supporting consistent methodologies in oncology drug development.
Paving the Way for the Next Generation of Cell and Gene Therapies
Accelerating Innovation and Access to Transformative Cancer Treatments
We are advancing cell and gene therapy development to ensure these life-saving treatments reach patients safely and efficiently. In collaboration with the Parker Institute for Cancer Immunotherapy (PICI), the FDA, and other key stakeholders, Friends mapped considerations on regulatory flexibility, clinical trial innovations, and patient-centered solutions to accelerate progress. By tackling scientific and regulatory challenges, we are shaping policies that expand access to next-generation therapies, bringing new hope to patients and transforming cancer care.
In 2025, Friends will explore key areas including new manufacturing models and innovative clinical trial designs to support individualized treatment paradigms and next-generation products.
ctMoniTR: Advancing ctDNA as an Intermediate Endpoint to Accelerate Cancer Drug Approvals
Validating ctDNA to Streamline Cancer Treatment Development
Friends is leading efforts to establish circulating tumor DNA (ctDNA) as an intermediate endpoint for use in clinical trials, helping safe and effective treatments reach patients faster. Through the ctMoniTR Project, we align data across clinical trials to demonstrate that changes in ctDNA reliably predict treatment response. In 2024, Friends published findings assessing baseline levels of ctDNA in real world datasets, presented research at the Society for Immunotherapy of Cancer (SITC) Annual Meeting, and discussed a framework for integrating ctDNA into clinical trials at our Annual Meeting. By advancing regulatory acceptance of ctDNA, we are reducing trial burdens and accelerating access to life-saving therapies.
In 2025, Friends will transition to the next step of the ctMoniTR Project to evaluate whether similar trends observed in earlier analyses extend across multiple cancer types and treatment modalities, with findings informing trial-level meta-analyses across prospective trials.

Friends Policy Priorities
Friends proposes unique approaches to solve current challenges in oncology drug development. The evidence and proposals generated from these efforts inform Friends’ legislative and regulatory policy priorities, which are organized into specific focus areas including:
- Advancing Drug Development
- Inclusive Clinical Research
- Diagnostic Harmonization and Development
- Innovative Trial Designs
Friends of Cancer Research Annual Meeting
Friends’ Annual Meeting identifies actionable opportunities to drive progress in cancer research and care. Over the years, our Annual Meeting expert working groups aligned on key concepts that informed white papers and provided the foundation for launching several collaborative research initiatives and supporting the development of regulatory policy and legislation.
The 17th Annual Meeting brought together leaders from federal health and regulatory agencies, academic research, the private sector, and patient advocacy to propose unique approaches to challenges in drug development.
- Enhancing Study Designs and Interpretation of Interim Overall Survival Data in Oncology Trials
- Pragmatism in Postmarket Trials
- Framework for Integrating Change in ctDNA Levels in Advanced Cancer Clinical Trials to Support Meta-analyses for Intermediate Endpoint Validation
In 2025, Friends will host our Annual Meeting on November 4 focused on emerging topics in oncology clinical trials and drug development.
Education and Outreach Programs
Friends continues to empower patients through our advocacy education programs ProgressforPatients.org and Project TEACH. These programs represent the standard for regulatory education, reaching nearly 1,000 patients, advocates, and caregivers, and connecting hundreds with future opportunities to add their voice to the drug development and clinical trial process. Get involved, access our courses free-of-charge, and share these programs with anyone interested.
Top Friends Downloaded Publications
Read Friends 2024 Scientific Report
In 2024, Friends made significant strides in advancing oncology drug development and regulatory policy. We compiled all our research and milestones from the past year into one report.
Click to access Friends’ 2024 Scientific Report.
Advisory Advocates
We are deeply grateful to our Advisory Advocates, who provide the patient perspective on our projects and messaging, creating a forum for sharing the needs of the patient community as it relates to cancer research, oncology drug development, and FDA regulatory affairs. Advisory Advocates are patients, caregivers of patients, and survivors of cancer who provide invaluable insights on their experiences to ensure patient perspectives are represented in Friends’ work. They volunteer to serve as project members on Friends’ projects and join meetings as panelists.
28th Cancer Leadership Awards
In 2024, Friends honored NCI Director Monica Bertagnolli with the Ellen V. Sigal Advocacy Award and Congressman Brett Guthrie with the Marlene A. Malek Public Service Award.
A special thank you to our 2024 Sponsors.
Friends’ 2024 Impact Report is a testament to your tireless commitment to our organization and the work we do every day for patients. On behalf of our organization, thank you. Your partnership and support in these important endeavors are crucial.
A special thank you to our Board of Directors, partner organizations, and supporters, who made this critical work possible.
Looking forward to working with you this year.

Ellen V. Sigal, PhD
Chair & Founder

Marlene A. Malek, RN
Vice Chair & Co-founder
Jeff Allen, PhD
President & CEO