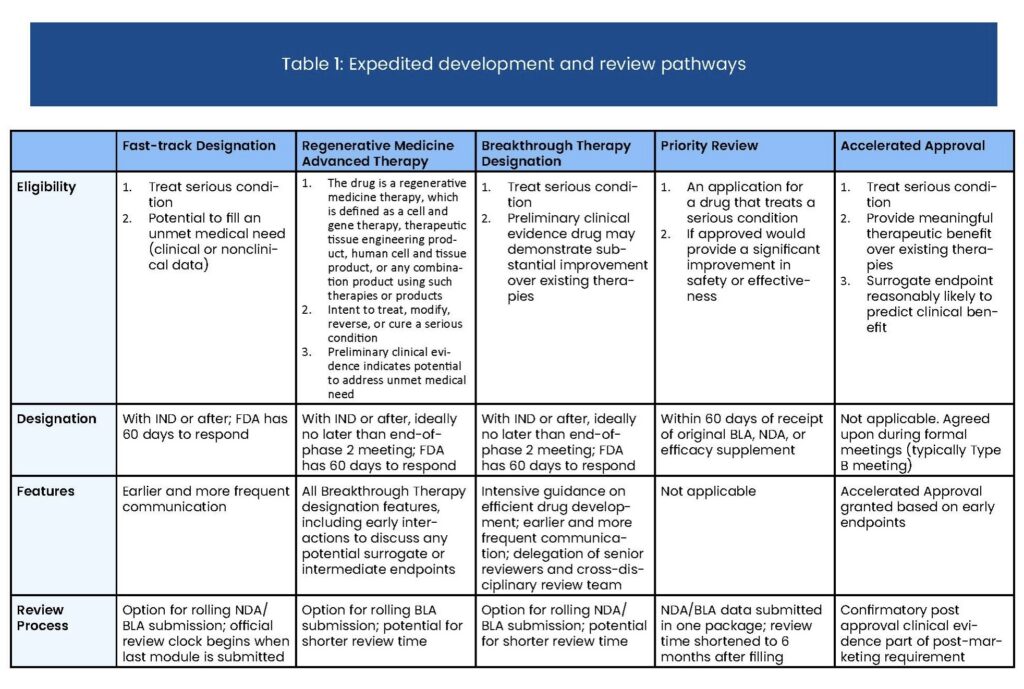
Regenerative medicine is a rapidly expanding field that has the potential to treat serious conditions, particularly in patients with unmet medical needs. CBER recognizes the importance of regenerative medicine therapies and is committed to ensure they are licensed and available to patients with serious conditions as soon as it can be determined they are safe and effective. The programs described in the guidance about RMAT are intended to facilitate development and review of regenerative medicine therapies intended to address an unmet medical need in patients with serious conditions.
Below are key points to understand about regenerative medicine advanced therapy (RMAT) designation:
- The request for RMAT designation must be made either concurrently with submission of an Investigational New Drug application (IND) or as an amendment to an existing IND.
- Regenerative medicine therapy includes cell therapies, therapeutic tissue engineering products, human cell and tissue products, or combination products using such therapies.
- Serious or life-threatening diseases are the intended targets for treatment with the designated therapy.
- Preliminary clinical evidence must show the potential of the drug to address unmet medical needs for the specified disease or condition.