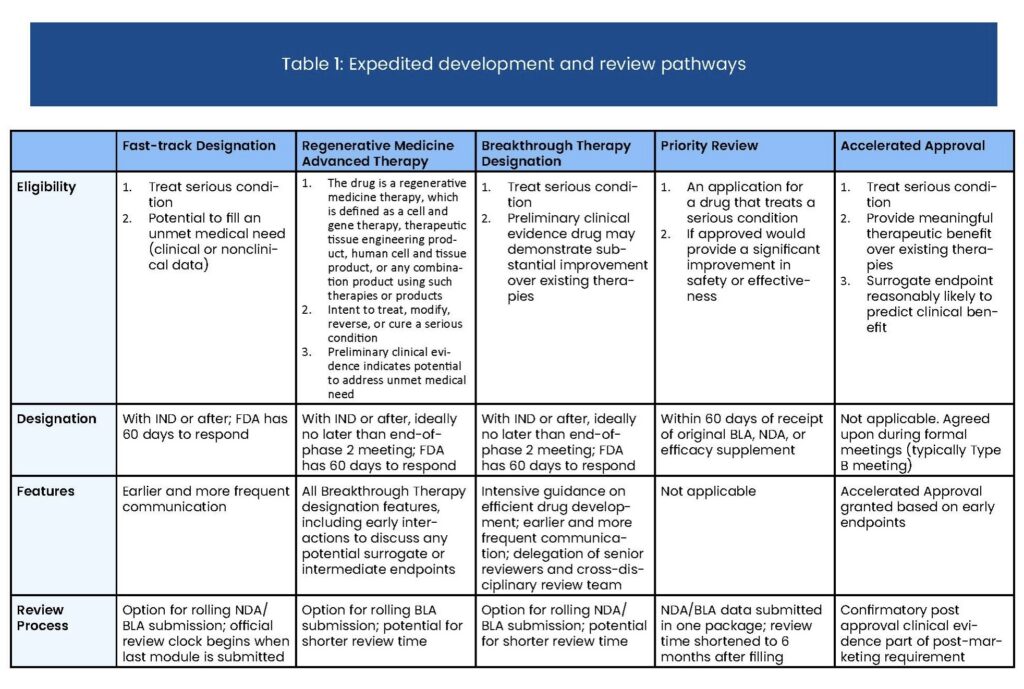
The FDA expedites the review of drugs treating serious conditions with the potential to provide significant improvements in safety or efficacy over existing therapies. Priority Review cuts the time in which the FDA will take action on a drug’s application from ten months to six.
Below are key points to understand about the Priority Review process:
- Priority Review Status is requested alongside Biologics License Application (BLA) or New Drug Application (NDA) submission
- The status cuts a drug’s FDA review period from ten to six months
- Drugs qualifying for Fast Track, Breakthrough Therapy, and Accelerated Approval can also be eligible for Priority Review