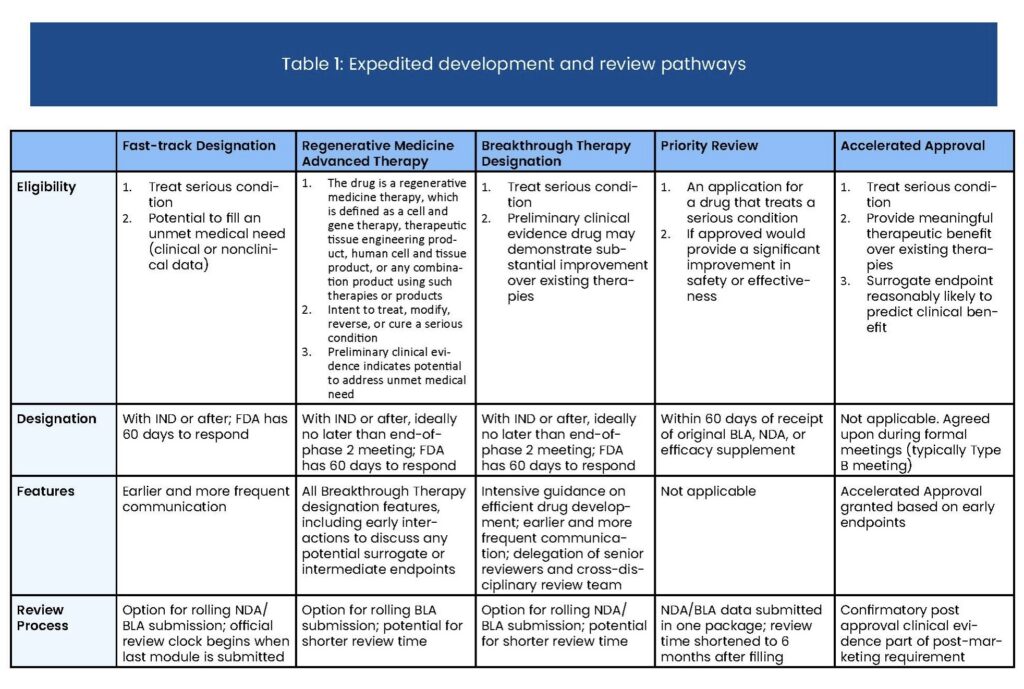
Fast Track is an expedited process that increases communication between drug sponsors and the Food and Drug Administration (FDA) throughout the development and review process for drugs that address unmet medical needs and treat serious or life-threatening conditions.
Below are key points to understand about the Fast Track process:
- The designation is requested as early as Investigational New Drug (IND) application and prior to BLA or NDA submission
- It is intended for drugs that address unmet medical needs by either treating a condition for which no other treatment exists or offering some substantial benefit over existing treatment
- Sponsors get extra opportunities to meet with FDA, discuss approval requirements and study design, and identify their most efficient path through drug development and review
- Sponsors may also gain access to rolling review, wherein portions of their marketing application may be reviewed before the complete application has been submitted.
Learn how this expedited pathway relates to others on our Expedited Pathways page.