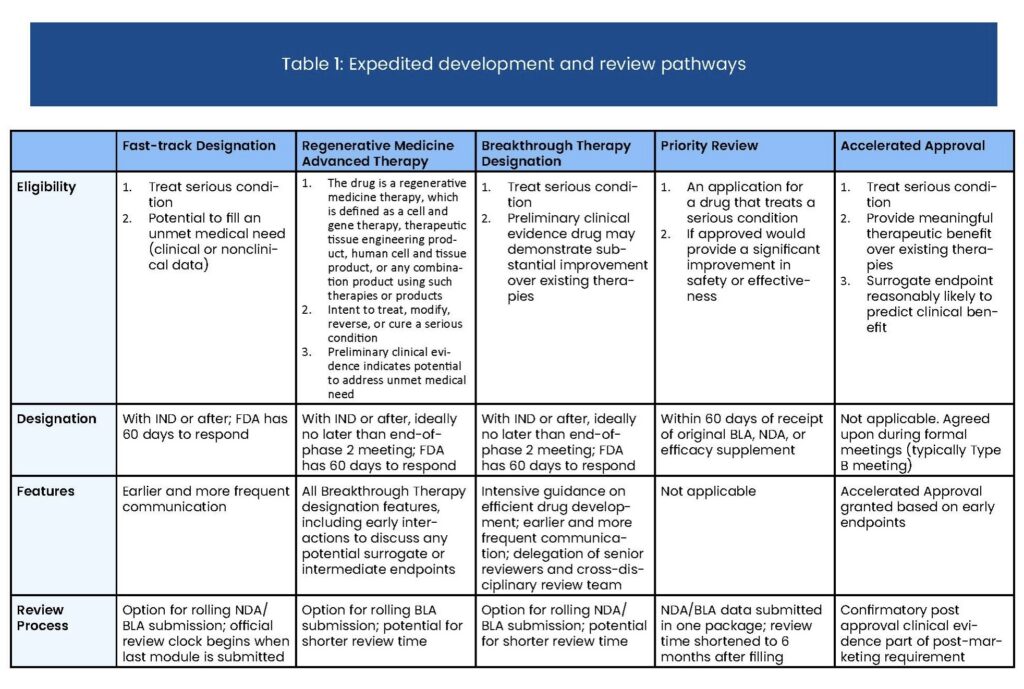
- Accelerated Approval
- Breakthrough Therapy Designation
- Fast Track
- Priority Review
- Regenerative Medicine Advanced Therapy (RMAT) Designation
The Food and Drug Administration (FDA) attempts to review all drugs efficiently, but gives special consideration to innovative therapies that treat serious or life-threatening diseases or have the potential to provide significant benefits to patients. The FDA uses four distinct mechanisms to speed the development and availability of drugs treating serious or life-threatening conditions: Priority Review, Accelerated Approval, Fast Track, and Breakthrough Therapy. While they all aim to improve FDA efficiency, these approaches employ different approaches and target different parts of the drug development and approval process.
Interested in exploring data on oncology drug approvals using an expedited program? Explore our dashboards here.
For a more comprehensive overview of these programs, read the FDA’s own description here.
Here’s a quick comparison of these programs: