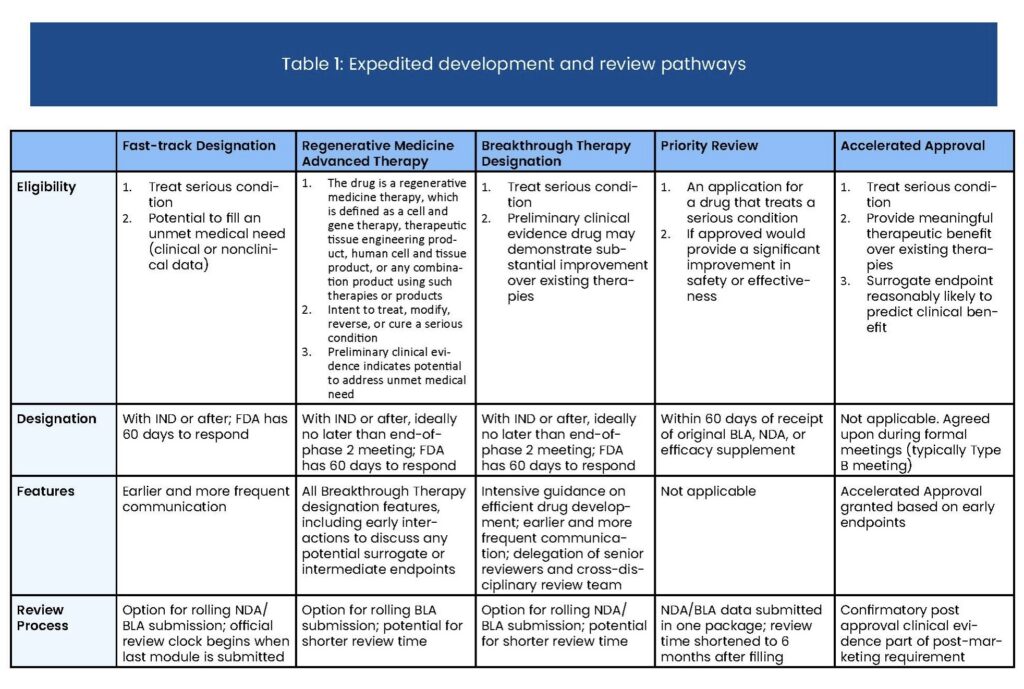
Breakthrough Therapy designation is granted for drugs and biologics that treat a serious or life-threatening condition and demonstrate early clinical improvement over existing therapies. Sponsors of Breakthrough drugs meet with Food and Drug Administration (FDA) officials more regularly than with a traditional approval pathway to ensure the most efficient possible path to approval.
The Breakthrough Therapy designation was established by the FDA through the Food and Drug Administration Safety and Innovation Act (FDASIA) in 2012, to speed up the development of drugs that offer significant benefits for serious or life-threatening conditions. In 2013, Friends helped conceptualize and implement the Breakthrough Therapy designation.
Below are key points to understand about the Breakthrough Therapy Designation process:
- Requested as early as IND application and preferably prior to end-of-Phase II meeting
- Similar to Fast Track, but Breakthrough drugs must show early clinical evidence of substantial improvement over existing therapies
- Same benefits as Fast Track, with additional opportunities for enhanced early meetings and coordination with experienced and senior FDA personnel
- Due to their large early clinical effect, Breakthrough drugs can sometimes skip portions of the standard FDA review process without compromising safety and efficacy standards
Search our database for medical therapeutics granted Breakthrough Therapy designation.