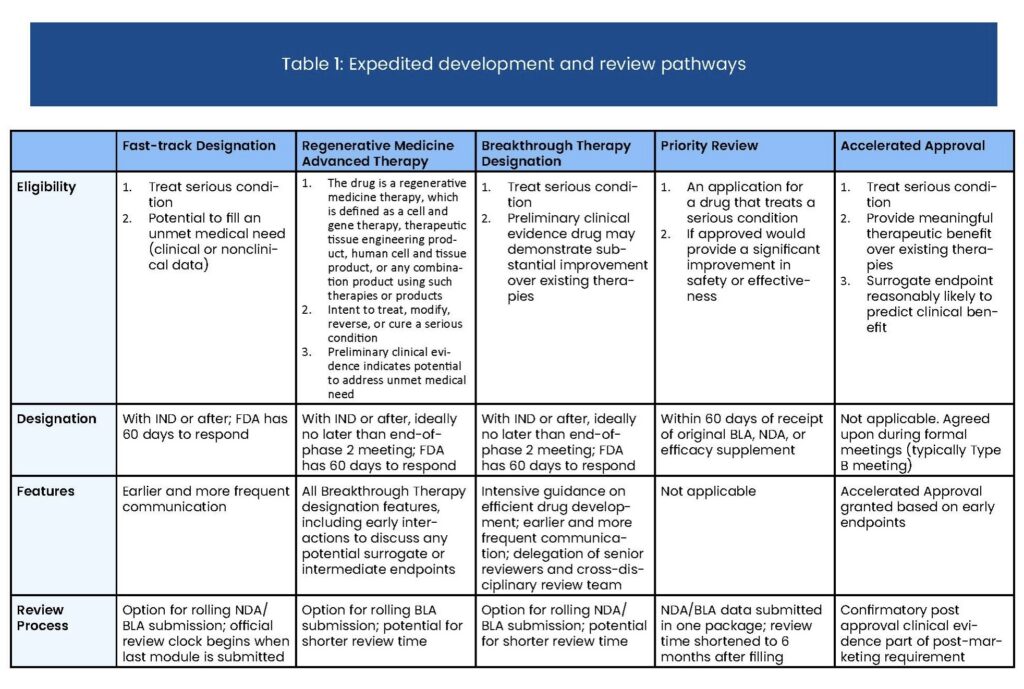
Many drugs that treat serious or life-threatening conditions lead to long-term clinical benefits, such as improved survival or decreased morbidity, that would take years to measure in clinical trials. The Accelerated Approval pathway allows the Food and Drug Administration (FDA) to approve certain drugs based on endpoints that can be measured earlier than overall survival, facilitating earlier access to therapies for serious, life-threatening diseases with unmet need. Sponsors are required to confirm the drug has clinical benefit through confirmatory post-market trials.
The Accelerated Approval regulations were first instituted by the FDA in 1992 in order to speed the availability of new drugs to treat HIV/AIDS. The approval pathway has also been frequently applied to cancer drugs and legislation passed in 2012 that codified the pathway into law.
Below are key points to understand about the Accelerated Approval process:
- Sponsor discussions with the FDA should occur during the drug development process.
- Drugs targeting long-term endpoints, such as increased survival or decreased morbidity, qualify when these outcomes are difficult to measure efficiently in clinical trials.
- Surrogate endpoints— more easily measured outcomes that are reasonably likely to predict clinical benefit (e.g., tumor shrinkage as a surrogate for survival benefit in some cancers)—allow for earlier approval.
- Obligations include confirming a drug’s efficacy in confirmatory post-market clinical trials.
- Priority Review, Fast Track, and Breakthrough drugs may also be eligible for Accelerated Approval