Diagnostics Harmonization Portfolio
Friends Diagnostic and Research Partnerships
Diagnostic tests play a crucial role in developing oncology drugs and determining the best treatment options for cancer patients. It’s important that these tests are reliable and consistent across the industry.
Friends Diagnostics Harmonization Portfolio focuses on standardizing these tests by generating evidence to understand variability, ensuring that different testing methods provide accuracy and consistency for patients.
Patients and clinicians may make major medical decisions based on potentially discrepant test results. Variability exists across diagnostic tests used for treatment decision-making. Alignment is needed to ensure results are consistent, accurate, and reliable no matter which test is used.
Scientific innovation supports the development of more effective and safer treatments for patients through the identification of specific signals known as biomarkers. Since not all cancer cells have specific biomarkers targetable by current therapies, it is necessary to identify which patients have the biomarker and may benefit from specific targeted therapies. To help identify which patients have a specific biomarker, diagnostic developers create tests, also known as assays.
Test developers use various approaches to identify a specific biomarker which can lead to variability in the results generated by different tests assessing the same biomarker. This lack of harmonization can make it challenging for patients and providers to interpret test results. Innovation in diagnostic testing, such as the use of computational methods for assessing tissue to aid in the identification of biomarkers has potential to improve test accuracy and reliability; however, these technologies have potential to introduce additional inter-test variability.
Friends’ diagnostic partnerships generate evidence to better understand factors that drive variability between tests critical in oncology drug development and informing care of patients with cancer.
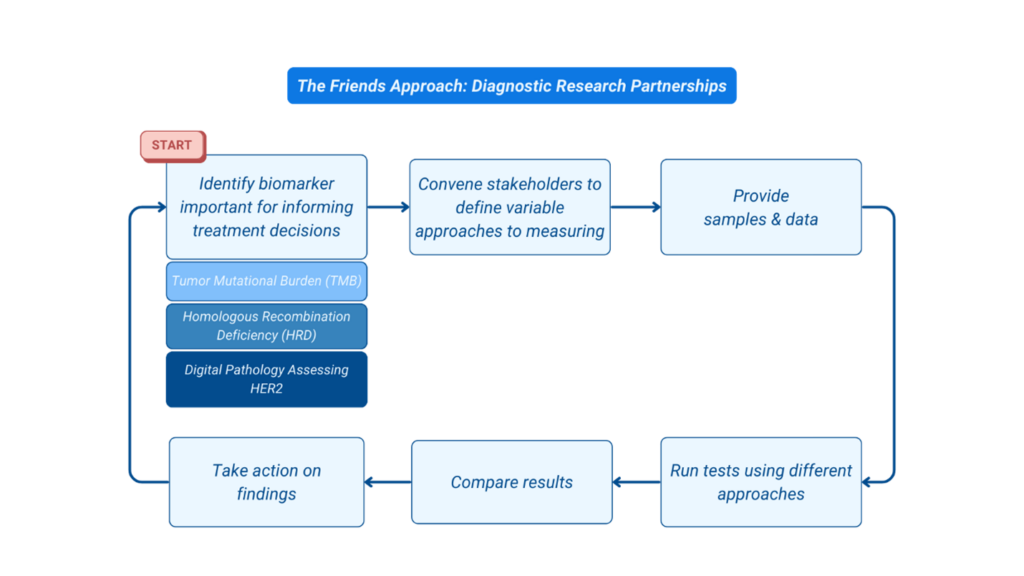
In collaboration with diagnostic developers, patient advocates, government officials, pathologists, and drug developers, we assess the comparability of biomarker measurements across different tests analyzing a common dataset, compare results, and identify opportunities to improve alignment.
Alignment across tests provides more consistent, accurate, and reliable results to inform patient and provider decision making.
Friends’ Diagnostic Research partnerships provide foundational information about the level of variability across assay outputs, establishing a baseline for patients when they consider their own test results. Without Friends coordination and support from collaborative sponsors, groups may never align on a solution to improve consistency across assays and interpreting results from assays would be more challenging for patients and providers.
Friends directs policy discussions and continues to support transparency in test performance and appropriate oversight of diagnostics.
Diagnostic tests, particularly Laboratory Developed Tests (LDTs), have historically been regulated under the Clinical Laboratory Improvements Amendments (CLIA), which primarily evaluates lab performance rather than the accuracy of the tests themselves. While FDA has maintained it has the authority to regulate LDTs as medical devices, the agency has historically exercised enforcement discretion to not evaluate most LDTs. Both the FDA and Congress have attempted to revise the current approach to regulating In Vitro Diagnostics offered as LDTs, seeking to modernize the outdated and inefficient diagnostic oversight process.
Friends diagnostic research partnerships provide insights into variability that can exist among diagnostics and offer viable solutions to enhance alignment and review. Friends remains deeply engaged in these policy discussions and continues to support transparency in test performance and appropriate oversight of diagnostics.
- 2023: Friends of Cancer Research Applauds Rep. DeGette and Rep. Bucshon for Introducing The VALID Act
- 2022: Friends publishes a white paper on “Expedited Development of Diagnostics for Therapies Targeting Rare Biomarkers or Indications.”
- 2022: Friends urges Congress to include the VALID Act as part of the 2023 omnibus appropriations bill.
- 2022: Friends supports the inclusion of the VALID Act in it in the Senate HELP user fee reauthorization bill.
- 2020: Friends thanks Senators Burr and Bennet and Representatives DeGette and Bucshon for their commitment to patients through the introduction of the VALID Act.
- 2016: Friends President & CEO testifies before congress on “Laboratory Testing in the Era of Precision Medicine.”

