Centers of Excellence
Centers of Excellence at the FDA: A Disease-Oriented Approach to Medical Product Development
History of the Oncology Center of Excellence
After spending several months analyzing the current regulatory environment and examining opportunities for potential improvement in the review process of new cancer treatments, Friends of Cancer Research (Friends) proposed to establish Centers of Excellence at FDA. As a first step, Friends proposed a pilot in oncology, which could be followed by Centers of Excellence in neurodegenerative disorders, cardiovascular health, and infectious disease. On June 29, 2016, the Vice President announced that an FDA Oncology Center of Excellence (OCE) will be created. The same day, FDA Commissioner, Dr. Robert Califf, issued an announcement naming Dr. Richard Pazdur the acting director of the OCE. To read Dr. Califf’s statement, please click here. On December 13, 2016, President Barack Obama signed the 21st Century Cures Act into law, including $1.8 billion for the Cancer Moonshot, formally establishing the Oncology Center of Excellence at FDA.
The Issue
Congress has not modernized FDA’s structure since the 1970s with the existing regulatory framework being defined by a “divide and conquer” approach to oversight. In order to reflect 21st Century cancer care, the agency should enhance coordination across its current centers to ensure consistency across the agency and facilitating optimal regulation of increasing complex medical treatments. Taking advantage and coordinating FDA’s broad expertise across major disease areas will help the agency adopt a patient-centered orientation to reflect the current multimodal approach to patient care.
Our Work Toward a Solution for Patients
During the past year, Friends worked with various stakeholders on taking our Centers of Excellence proposal from an idea first appearing in an op-ed in The Hill to becoming a reality announced at Vice President Biden’s Cancer Moonshot Summit on June 29, 2016. In addition to the op-ed, Friends held a Congressional briefing, which allowed experts to discuss how FDA could refocus its resources and ensure the best outcomes for patients to organizing a sign-on letter with 27 other organizations asking FDA Commissioner Dr. Robert Califf to create the OCE. Now, the OCE is a reality at the FDA with the passage and signing of the 21st Century Cure Act in December, 2016.
Key Considerations
- Exciting new therapies are changing the way we prevent, diagnose and treat cancer – rarely do patients receive one drug, but require multi-model care.
- Advances in science are offering patients new and innovative treatment options, but traditional regulatory processes have become more complicated with the increased reliance on combinations of therapies, genomics, diagnostic tests, and precision medicine.
- Numerous parts of the regulatory system need to work together to ensure patients have access to the right treatment, at the right time.
- Regulators at FDA need the proper tools at their disposal to reliably and appropriately protect public health and ensure that their decisions are made in the full context of cutting-edge medical care.
Key Concepts for Centers of Excellence
- Introduce organizational enhancements for medical products that involve multiple FDA centers such as the development of companion diagnostic tests, as well as the use of combinations of drugs, biologics, and devices to treat cancer.
- Reorganize FDA processes by taking a step-by-step approach, ensuring the agency can continue to effectively monitor and evaluate the safety and effectiveness of treatments for patients with limited disruption during this process.
- Involve industry experts, regulatory agencies, patient advocates, academics and medical professionals to explore optimal activities and processes for the Center of Excellence.
- Develop and facilitate new approaches to promote the science of precision medicine.
- Provide additional resources to FDA for implementation of the Centers of Excellence.
- Ensure the U.S. remains the gold standard for medical innovation.
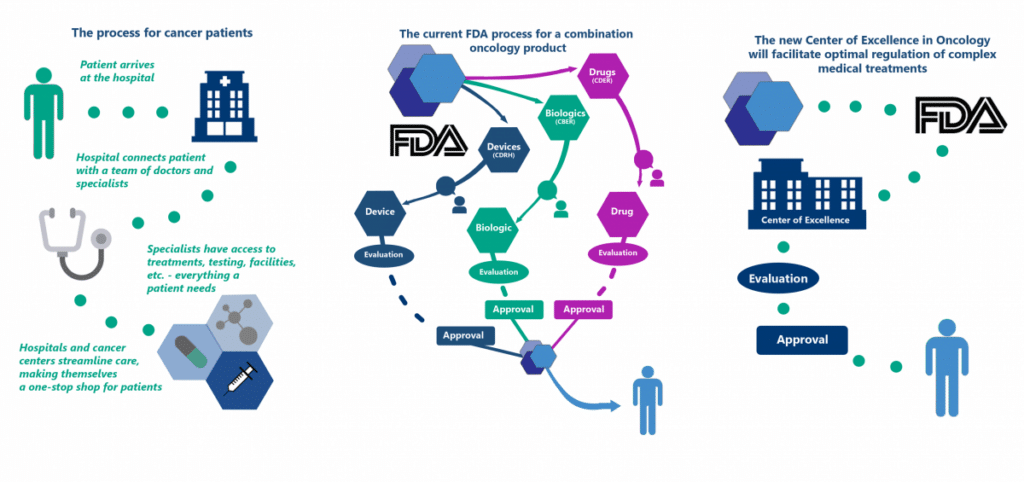
Streamlining the Review Process for Regulators to Benefit Patients
When a cancer patient goes in for treatment at a hospital or treatment center, their care is streamlined through a collaborative process where doctors and specialists work together to treat the disease as a whole. In contrast, when a combination oncology product is submitted to FDA for review, it is divided into individual components and split between FDA’s medical product divisions. Each division makes separate approval decisions on different review timelines, resulting in a cumbersome process for patients, regulators, and drug makers. The graphic below illustrates how the new FDA Oncology Center of Excellence will facilitate optimal and efficient regulation of these complex medical treatments. This in turn will promote the science of precision medicine and innovative treatments in oncology to benefit patients in need of life-saving therapies.