Friends of Cancer Research (Friends) had an eventful year of new projects launched, data insights shared at public meetings and in publications, and partnerships formed. For the final 2024 edition of the advocate newsletter, we’re sharing three advocate’s perspectives on our Annual Meeting topics and reflecting on progress made this year. We look forward to what’s ahead in 2025!
Here’s what you can read more about in this newsletter:
- What’s Happening at Friends?: Read three advocate’s perspectives on our annual meeting topics.
- Did You Know?: Read about notable advancements in oncology therapies approved this year by FDA.
- Mark Your Calendars: Friends of Cancer Research is hosting a few upcoming meetings: one on advancing diagnostics on February 4th with a follow-up diagnostics webinar for advocates on February 19th, and another on next-generation therapies on May 9th.
- Important News In Cancer Research: Recent news, FDA activities, and policy developments highlighting topics related to Friends’ work and topics impacting cancer care and drug development.
- 2024 Year in Review: Recap of our advocate program this year.
What's Happening at Friends?
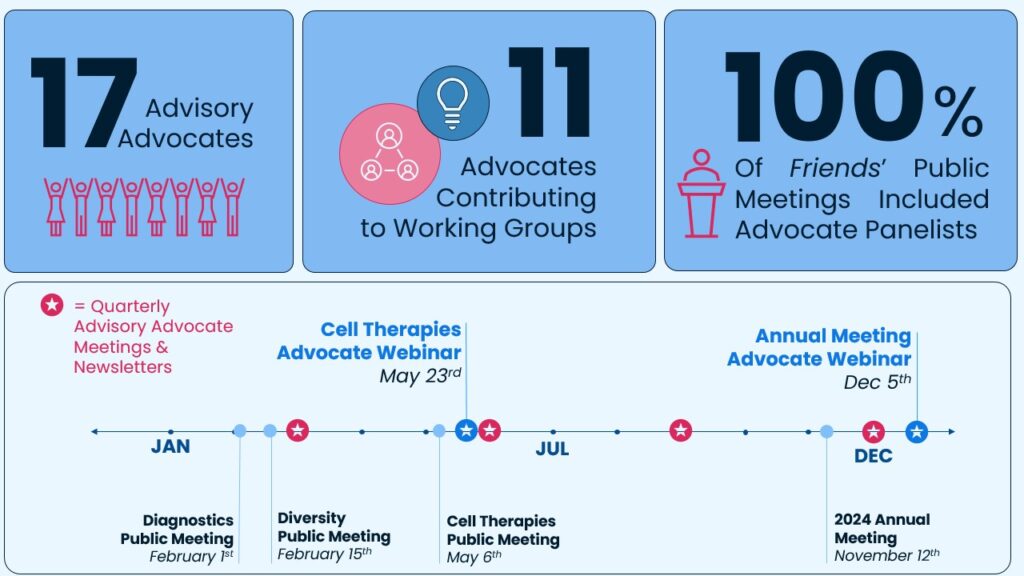
Friends held our 2024 Annual Meeting on November 12th in Washington, D.C. The meeting was a culmination of the collaborative efforts of scientists, clinicians, advocates, and policymakers to develop innovative solutions to challenges in oncology drug development. The panel discussions during the meeting focused on three topics: interim OS evaluations, pragmatic trials in the post–marketing setting, and incorporating ctDNA in prospectively designed trials. During the panels, we heard from three patient advocates who shared their insights, which helped to shape recommendations included in the meeting white papers and will inform next steps toward advancing oncology drug development. These contributions are highlighted below.
“I don’t want drugs delayed because immature overall survival (OS) data interferes with it getting accelerated approval…The FDA and the sponsor have to reach an agreement on whether or not interim OS data belongs in the picture because it may not. It may be inappropriate. It just may be too small, too immature… I think pre-specification of when and how to use interim OS is so important because I don’t want it introduced if it’s not useful. If it’s just confounding or weak enough that we shouldn’t draw conclusions from it, then it doesn’t belong in the discussion at the time accelerated approval is sought. Leave it out for fear that we err on either side and continue to gather data.”


“I really like the idea of starting with the post-market setting for [pragmatic trials] because I think it gives us an opportunity to build truly patient-centered research. Because we know that the drug is safe and effective for a specific use, but there are a number of unanswered questions… [post-market studies] give us a chance to improve the patient experience of trial participation by adjusting the protocol to make it as streamlined as possible and finding additional ways to reduce patient burden and engage and enroll the needed participants. It also gives us the opportunity to do implementation strategy testing because we’re still in a research environment. We can really look at how to best generate awareness and interest, and ultimately uptake of the improved treatment. And that’s what makes me very excited about this.”
“I think it’s important to look at [the feasibility of measuring ctDNA] from the burden or the incentive patients will have to be in this study. We know people need to go to a clinic for their treatments, and if we can align the ctDNA collection with that schedule I think we have a much better chance of having people enroll and stay with the program. I live in a rural state and sometimes people need to drive for several hours to get their treatment. They may need to have someone drive them, there may be childcare issues, time away from work, all of that. And to carve out more time for yet another test, if it’s not on that cycle, would be burdensome to participants. So, I think the more we can align and make it less of a barrier for patients, the more we can enroll folks in the trial and keep them as participants.”

As a follow-up to the Annual Meeting, on December 5th, we hosted a recap webinar for advocates to dive deeper into these topics and to provide a forum for advocates to ask additional questions. If you missed it, we encourage you to re-watch the discussion!
Did You Know?
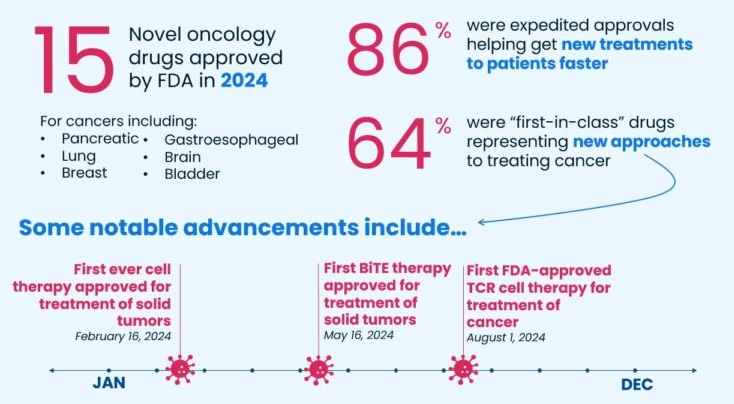
The U.S. Food and Drug Administration (FDA) approved several novel cancer therapies in 2024, representing new treatment advances for cancer.
Mark Your Calendars
Please register for our upcoming events at the links below.
🟩 = Free | 🔷 = Friends’ Event
**Look out for emails with additional event details!
Important News in Cancer Research
📰 The News: The FDA created a new Digital Health Advisory Committee (DHAC) to provide advice and recommendations around use of digital health technologies, such as medical devices that incorporate artificial intelligence (AI). In November, the committee met for the first time to discuss unique regulatory needs for generative AI-enabled medical devices. Read more here.
| 💡 Why it’s important: AI-enabled devices have potential to enhance clinical practice by providing more accurate results and facilitating more efficient processes; however, there are unique risks associated with these new technologies. The two-day DHAC highlighted industry, regulator, patient, and academic perspectives on the risks and complexities of GenAI-enabled devices that challenge current regulatory approaches to evaluation and monitoring, and evidence generation. In oncology, AI is being used to aid biomarker and tumor assessments. Friends is leading efforts, such as our Digital PATH Project, to support robust development of AI-enabled technologies used in oncology drug development. Initial findings from the project were presented during the San Antonio Breast Cancer Symposium in December. |
📰 The News: In November, FDA finalized a guidance document on “Use of ctDNA for Curative-Intent Solid Tumor Drug Development.” | 💡 Why it’s important: This guidance outlines recommendations for using ctDNA in drug development. The document provides considerations that will guide future use of ctDNA as an early- endpoint in clinical trials, and aligns with our ongoing work, including this year’s annual meeting topic to inform a framework for incorporating ctDNA as an early-endpoint in oncology clinical trials and our ctMoniTR Project. |
📰 The News: In September, FDA issued a new draft guidance on “Considerations for Generating Clinical Evidence From Oncology Multiregional Clinical Development Programs.” | 💡 Why it’s important: Many oncology clinical development programs and clinical trials are conducted at sites around the world which can impact the applicability of data to U.S. populations due to variation in standard of care practices, treatment patterns, and patient characteristics. This guidance provides considerations to guide planning for global trials and will help to ensure data submitted to support FDA’s regulatory decisions are representative of and applicable to U.S. patients and the U.S. healthcare system. Read our comment on the guidance here. |
2024 Year in Review
In 2024, we continued to expand our Advisory Advocate community, launched a new advocate-focused webpage, and started hosting advocate-focused webinars to provide additional opportunities to engage on our meeting topics.
A special thank you to our Advisory Advocates who have contributed their expertise and insights to inform our work this year.
We look forward to continuing progress and fostering new partnerships in 2025!