
Welcome to Friends’ third Quarterly Advocacy Newsletter of 2024. In Q3, Friends published several peer-reviewed manuscripts with insights on enhancing representation in clinical trials, use of RWD to assess response to treatment, and opportunities for pragmatic elements to be incorporated in clinical trials supporting regulatory decisions. We’re excited to share details on these publications and more in this newsletter:
Here’s what you can read more about in this newsletter:
- What’s Happening at Friends?: Read about the role of peer-reviewed publications in advancing science and see how 3 of Friends’ recent peer-reviewed papers contribute to advancing oncology research and policy.
- Did You Know?: Read how a recent Supreme Court decision could affect how federal agencies, such as the FDA, make regulatory decisions and how this could impact patients.
- Mark Your Calendars: Friends of Cancer Research Annual Meeting 2024 on November 12th and additional advocacy opportunities.
- Advocate Spotlight: Hear from a patient advocate about how ProgressforPatients.org played a role in their advocacy journey.
- Important News in Cancer Research: Recent news, FDA activities, and policy developments highlighting topics related to Friends’ work and topics impacting cancer care and drug development.
What's Happening at Friends?
SAVE THE DATE! Friends Annual Meeting is on November 12. The meeting will be held in-person in Washington, D.C. and streamed virtually – it’s a chance for us to discuss three key topics that broadly focus on approaches for streamlining trial designs and data collection to support efficient regulatory processes and decision-making. For each of the three topics, we’ll share a white paper ahead of the meeting as a primer for the topic, then have panel discussions about each. Also save the date for a patient advocate recap discussion on Thursday, December 5 during which we’ll review topics discussed during the Annual Meeting and answer your questions. You don’t want to miss it!
Friends’ Research Publications
Below we highlight three of Friends’ recent peer-reviewed publications and their implications for cancer research and policy.
An Evaluation of Novel Oncology Approvals with a PMR/C for Assessing Data in Racial and Ethnic Populations Underrepresented in Premarket Clinical Trials | Published June 2024 in Clinical Cancer Research This publication shares an analysis of postmarketing studies for recently approved novel oncology drugs and explores characteristics of the drug and clinical trial design that led to approval that may prompt a postmarket study for evaluating data in a more racially and/or ethnically diverse population. These insights can guide the design of future pre-market clinical trials to ensure they evaluate safety and efficacy in representative populations to support an understanding of any differences before approval. |
Evaluation of real-world tumor response derived from electronic health record data sources: A feasibility analysis in mNSCLC patients treated with chemotherapy. | Published August 2024 in ASCO’s Journal of Clinical Oncology (JCO) Clinical Cancer Informatics This publication details a collaborative effort among real-world data (RWD) providers, pharmaceutical companies, academics, researchers, and government experts. It evaluates seven RWD sources to determine the availability of RWD and the ability to assess patients’ responses to treatment using electronic medical records. The publication reveals that medical records can be used to assess treatment response in an aligned manner across data sources and offers a framework and future directions for using this information in regulatory decision-making. |
Bridging Research and Practice: Enhancing Regulatory Decisions with Pragmatic Clinical Trials in Oncology | Published August 2024 in European Society for Medical Oncology (ESMO) Real-World Data and Digital Oncology Pragmatic clinical trial elements may streamline data collection to ease the burden on patients and investigators and broaden eligibility criteria for more inclusive trials. To ensure trials are rigorous enough to support regulatory decisions, these elements must be carefully integrated into clinical trials. This paper offers insights on using a hybrid approach to better reflect real-world conditions while ensuring the data are suitable for regulatory use. These insights can be used to support future designs of pragmatic clinical trials. Our Annual Meeting this year we will further discuss opportunities to use pragmatic clinical trials in oncology drug development and regulatory decision-making. |
Did you know?
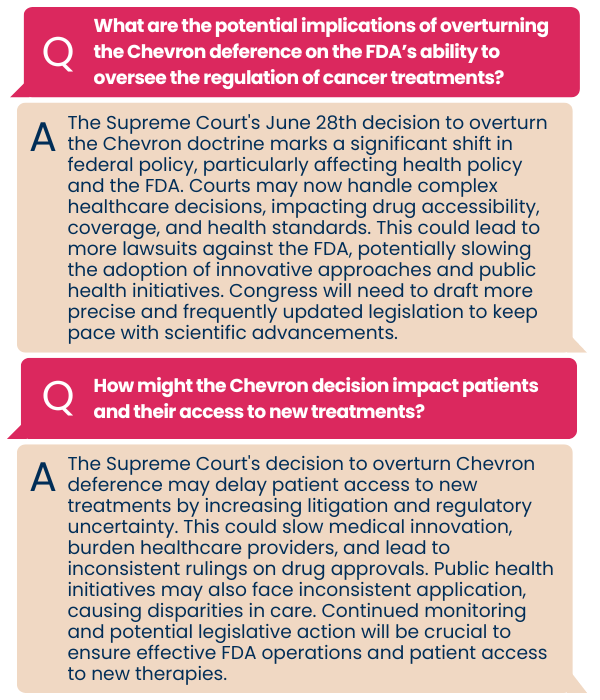
In June, the Supreme Court of the United States (SCOTUS) issued a ruling that reduces the power of federal agencies, such as the FDA, to interpret and enforce unclear laws. We asked Friends’ VP of Public Affairs, Ryan Hohman, to break down what this case means for cancer research and the FDA.
Mark Your Calendars
⬇️ = Reduced Advocate Registration | 🟩 = Free | 🔷 = Friends’ Event
| 🟩 September 18 – 19th, 2024 | Rally for Medical Research |
| ⬇️ September 21-24, 2024 | 17th AACR Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved |
🟩 October 29th, 2024 | MDIC Annual Patient Summit: Insights on Patient Science Tools in Evidence Generation |
| 🟩 🔷 November 12, 2024 | Friends 2024 Annual Meeting |
| 🟩 🔷 December 5, 2024 | Friends Annual Meeting 2024 Recap: Sharing Next Steps and Opportunities with Advocates |
| ⬇️ December 10-13, 2024 | San Antonio Breast Cancer Symposium 2024 |
Advocate Spotlight
Watch the video below to hear from a patient advocate about how ProgressforPatients.org informed their advocacy journeys.
Important News in Cancer Research
📰The News: FDA released three new draft guidance documents which provide recommendations for broadening cancer clinical trial eligibility criteria. | 💡Why it’s important: Eligibility criteria define which patients can be enrolled to a clinical trial. Cancer clinical trial eligibility criteria tend to be overly restrictive and unnecessarily exclude patients from participating in the clinical trial. As a result, clinical trial populations may not reflect the real-world population of patients who may end up receiving the drug following approval. To address this, the FDA has issued a series of guidance documents with recommendations for broadening certain commonly used eligibility criteria. In 2021, Friends worked with ASCO to develop recommendations for broadening cancer clinical trial eligibility criteria and these recommendations align with those included in the recent guidance. |
📰The News: FDA finalized guidance on “Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases.” | 💡Why it’s important: While the optimal dose for chemotherapies is usually established using a “more is more” approach to identifying the maximum tolerated dose (MTD), newer targeted oncology therapies are often just as effective at lower and less toxic doses that are more tolerable for patients. This has required a new framework for assessing doses and identifying the optimal dose. To support this, FDA finalized guidance that outlines expectations for dosing studies supporting oncology approvals. Friends submitted a public comment on the draft version of this guidance, released in 2023, which included recommendations informed by our working groups on tolerability and dosing. |
📰The News: FDA published a long-awaited guidance on Diversity Action Plans to help sponsors set goals for enrolling representative trial populations. | 💡Why it’s important: In 2022, Congress passed legislation that provides FDA with the authority to require sponsors to submit plans that set representative enrollment goals for clinical trials. These plans aim to ensure sponsors are actively working to include historically underrepresented populations in clinical trials supporting medical product approvals. This guidance is a critical next step toward ensuring clinical trials are representative of all patients who may use a therapy or device. Friends plans to submit a public comment on the guidance, and we encourage patient advocates to make their voices heard too. You can access other public comments, read the guidance, and submit a comment by September 26 here. |

