Summary
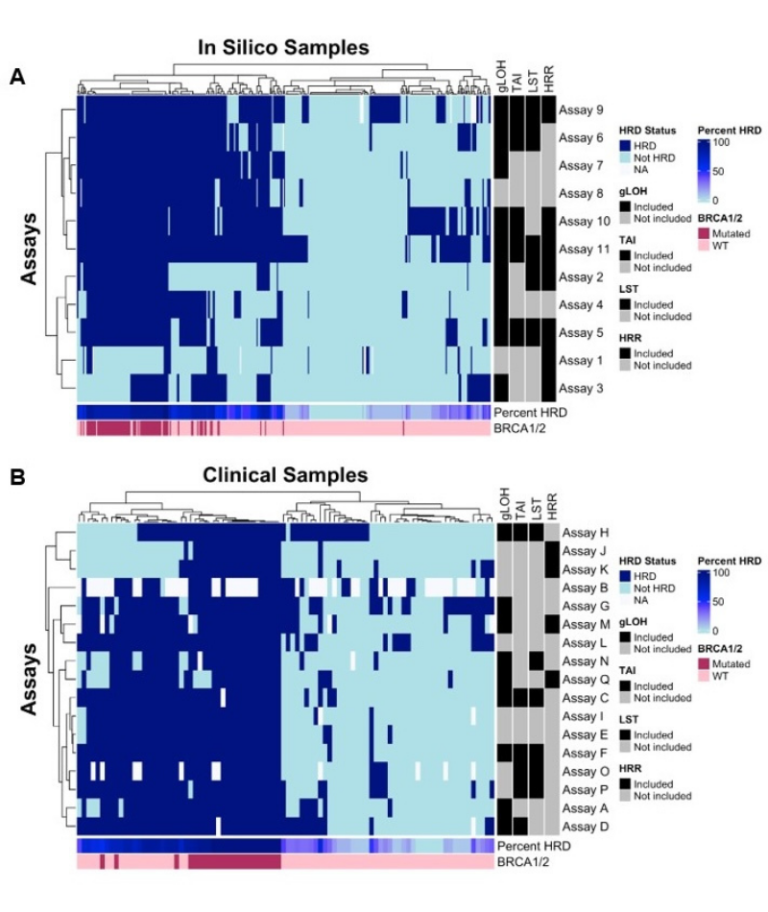
Friends‘ recent research on homologous recombination deficiency (HRD) involved a comparative analysis of 20 independent HRD assays, assessing both an In Silico (n=348; TCGA database) and a Clinical dataset (n=90; nucleic acids from freshly extracted archival ovarian cancer tumor samples). The assays measured a variety of outputs to define DNA damage repair dysfunction and identify patients with high-grade serous ovarian carcinoma (HGSOC) who had HRD. This is important because patients with HRD may benefit from DNA damage repair inhibitor treatments like Poly ADP-ribose Polymerase Inhibitors (PARPi).
The findings demonstrated discordance among HRD assays in both In Silico and Clinical analyses. When assessing assay factors, variability in HRD status call was observed in both datasets. The median pairwise positive percent agreement (PPA) for HRD in the In Silico samples was 74% (IQR 51-89%) and for the Clinical samples was 83% (IQR 70-91%). The median pairwise negative percent agreement (NPA) for the In Silico samples was 81% (IQR 64-92%) and for the Clinical samples was 80% (IQR 62-91%). While the sample size for the clinical analysis (n=90) was not powered to reach definitive conclusions, the analysis enabled the identification of trends and areas for future investigation to establish reliable benchmarks and to align on a defined reference standard to improve assay agreement.
Why This Research Matters
HRD diagnostic tests hold promise as critical tools for identifying patients who may benefit from DNA damage repair inhibitors, such as PARPi. By generating data that characterizes the variability HRD status across different assay outputs, this research, along with expanding body of literature on the subject, supports the development of more harmonized assays. Without a defined reference standard for HRD assay outputs, establishing clear agreement levels remains a challenge. The Friends HRD Harmonization Project aims to bring greater clarity to diagnostic test development and assessment, which is essential for improving the outcomes of patients with cancer and the advancement of novel therapies.
HRD assays measure DNA damage repair dysfunction to identify patients with ovarian cancer who could potentially benefit from drugs like PARPi, stated Dr. Ethan Sokol, Principal Scientist, Foundation Medicine. “It is important that all HRD assays provide optimal clinical performance for predicting treatment response.
While the analysis set the groundwork for what needs to occur to create alignment, we did not focus on a gold standard due to a lack of agreement on what that might be, said Dr. Lisa McShane Associate Director, Division of Cancer Treatment and Diagnosis at the National Cancer Institute (NCI). For all diagnostic development, it is important that gold standards and reference datasets are established to improve assay output consistency.
Thank you to our coauthors: Hillary Andrews, PhD, Lisa McShane, Elis C. Kohn, Rebecca Arend, Chris Karlovich, Kaitlyn Kincaid, Douglas Laird, Ming-Chung Li, Ethan Sokol, Elizabeth R. Starks, Shulin Bi, Lauren Brunner, Alyssa Chapman, Li Chen, Tommaso Coletta, Yuan Ding, Bailee Dover, McKenzie Foxall, Mohit Gupta, Zan Halford, Andrea G. Kahn, Nikita Kotlov, Yi-Hsuan Lucy Lai, Alexander J. Lazar, Wenjie Li, Brittany Avin McKelvey, Hyunjun Nam, Sarabjot Pabla, Pegah Safabakhsh, Daniel Saul, Albrecht Stenzinger, Timothy J. Taxter, M.D., Zhiwei Zhang, Yingdong Zhao, ShiPing Zou, Mark Stewart, Jeff Allen. This work is possible through a large-scale partnership.
Thank you to the following partners: Friends of Cancer Research, National Cancer Institute (NCI), University of Alabama at Birmingham, Frederick National Laboratory for Cancer Research, Pfizer, Foundation Medicine, Invitae, Amoy Diagnostics, PathAI, SOPHiA GENETICS, Illumina, Thermo Fisher Scientific, Diagnostic Laboratory Services, BostonGene, ACT Genomics, The University of Texas MD Anderson Cancer Center, Burning Rock Dx, NeoGenomics Laboratories, Labcorp Oncology (OmniSeq), Guardant Health, Bionano Genomics, Inc., University Hospital Heidelberg (UKHD), Tempus AI, Pillar Biosciences Incorporated.

