While significant progress has been made in cancer treatment and care, this progress has not always easily translated to new treatments for pediatric oncology patients. In 2003, the Pediatric Research Equity Act (PREA) passed requiring the study of cancer therapeutics in certain pediatric cases. Specifically, PREA required sponsors of new drug applications (NDAs) or biologics license applications (BLAs) to submit assessments regarding appropriate formulations for pediatric patients. However, PREA waived the requirements for those therapeutics with a non-pediatric relevant indication and provided exemptions for therapeutics with an orphan designation. This orphan designation exemption limited how many oncology therapies were required to complete pediatric studies since 75% (103/137) of NDAs and BLAs for oncology received an orphan designation since 2013. In an effort to require further pediatric studies, the Research to Accelerate Cures and Equity (RACE) Act was signed into law in 2017, which required pediatric studies for all new adult oncology therapeutics if the molecular target is relevant to pediatric cancer, including therapeutics with an orphan drug designation.
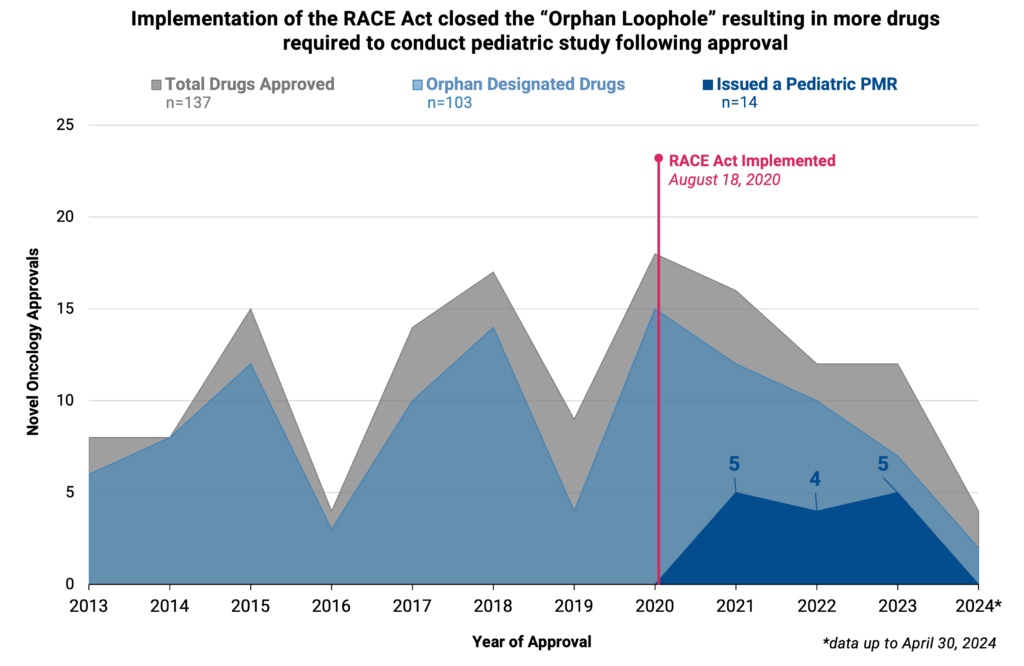
It has been nearly four years since the implementation of the RACE Act (implemented on August 18th, 2020). We assessed the impact of the Act on required pediatric studies and evaluated all new oncology NDAs and BLAs that were submitted and FDA approved since implementation (August 18, 2020 to April 30, 2024). Thirty-six therapeutics were identified within the study period, and since implementation, 41.7% (15/36) of oncology original approvals were required to conduct a pediatric study (n=14) or were approved for pediatric use at the time of initial application with no further required pediatric study (n=1).
Closing the orphan designation loophole through the RACE Act was effective in increasing pediatric study, as 71.4% (10/14) of drugs submitted or approved between implementation and April 30, 2024 with a required pediatric study were orphan designated therapies. Additionally, several approved therapies for adult cancer indications (e.g., breast, cervical, and lung cancer) were required to conduct pediatric study due to the mechanism of action being on the molecular target list, whereas prior to RACE the therapies would have been waived from study due to the non-pediatric indication. All required pediatric studies were deferred until after approval, with 17 postmarket requirements (PMRs) for the 14 therapies. The results of these studies and impact on pediatric access will not be known for some time, as the median time from drug approval to the PMR required final report is 5.96 years (range of 1.42 to 8.26 years). The majority of PMRs (70.6%, 12/17) are pending (i.e., the study has not yet been initiated but does not meet the criterion for being delayed). Only 17.6% (3/17) of PMRs are ongoing (i.e., the study has been initiated).
Analyzing the drug approvals where a pediatric study was waived or exempt (n=21) after RACE implementation highlights there are still inherent challenges with conducting studies in pediatric oncology patients. Many pediatric study requirements are still waived for therapeutics with a relevant mechanism of action for pediatric patients, due to studies being deemed “impossible or impracticable” (61.9% of agents, 13/21) because of the low prevalence of the cancer type or molecular target. This highlights the need for further innovative strategies to address challenges in clinical research for pediatric oncology patients, including through the encouraged use of master protocols and tissue agnostic trials, extrapolating certain safety and efficacy data from adults, and including at least adolescents in the registrational trial for relevant cancers.
Overall, the RACE Act has significantly increased pediatric study since its implementation. There were no PMRs for pediatric studies for new oncology drugs approved in the four years before RACE implementation. Longitudinal studies are needed to determine if any of the required pediatric studies result in label expansions for pediatric indications, the ultimate intended benefit of the RACE Act. Further innovative clinical trial strategies are needed to support study in rare pediatric patient populations.
This analysis was possible in part through data available in Friends’ interactive PMR/Cs dashboard, which includes data on PMR/Cs issued at the time of initial approval for novel oncology therapies between January 1, 2012, and December 31, 2023. Access the dashboard here.

