On June 22, 2020, Friends of Cancer Research (Friends) published a paper, “Use of Patient‑Reported Outcomes to Understand & Measure the Patient Experience of Novel Cell and Gene Therapies” in Therapeutic Innovation & Regulatory Science (TIRS) , a scientific journal of the Drug Information Association. In May 2018, the Centers for Medicare & Medicaid Services (CMS), initiated a National Coverage Determination (NCD) for Chimeric Antigen Receptor T cell (CAR-T) Therapies after FDA approval of the first two CAR-T therapies in oncology. CMS requested input from the Medicare Evidence Development & Coverage Advisory Committee (MEDCAC) and public feedback on how to include Patient Reported Outcomes (PROs) in future clinical studies, specifically for CAR-T therapies.
CAR-T is a type of cell therapy where live cells are taken from the patient and genetically modified to fight cancer cells. This therapy is expected to extend median overall survival by several years. In the space of gene and cell therapies, PROs are an important metric for advising coverage determination and long-term assessment, in part, because they are a valuable benchmark for evaluating patients’ experience of treatment in oncology. PROs offer ongoing surveillance to inform long-term value of treatments as curative therapies extend life well past the time of a clinical trial.
The final NCD for CAR-T did not include PRO collection as a requirement for coverage to ensure broad patient access. However, the consideration of PROs by CMS recognized the usefulness of these tools to enhance our understanding of long-term value for cell and gene therapies. In response to the proposed NCD, a multi-stakeholder group, convened by Friends, met to discuss inclusion of PROs as a factor in coverage decisions for CAR-T therapies. This paper advocates for the use of PROs to inform payer decisions for cell and gene therapies and outlines recommendations for standardized collection.
Friends developed a PRO collection framework, including a standardized approach to PRO collection, which is necessary to support quantitative assessment of patient experience. The paper outlines three important aspects that support utilizing PROs in payer decision-making.
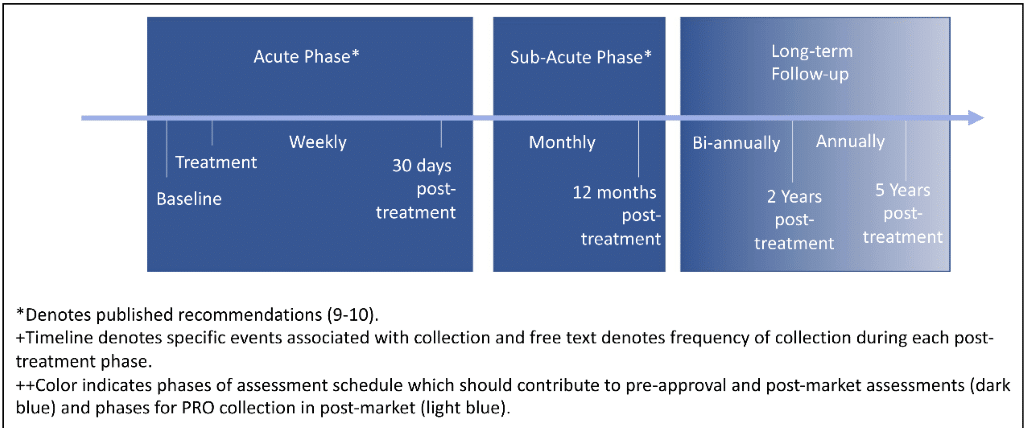
First, standardized PRO collection throughout all three phases of post-treatment follow-up is necessary to fully characterize the patient experience in response to CAR-T therapy. Second, an adequate infrastructure, including ePROs is necessary to support long-term PRO collection especially as patients transition to outpatient or routine care settings, a critical point in data collection where patients can be lost to follow-up. Real-world evidence suppliers and third-party vendors can provide additional resources to support continuous data collection. Third, use of electronic PRO capture is key to reducing burden for PRO collection and facilitating broad use of PROs for collecting evidence
Friends supports policies to accelerate advancements for curative therapies and collecting evidence to ensure access to gene and cell therapy products. Given the curative potential and long-term implications of treatment with CAR-T therapies, PRO collection will be an important tool to ensure the delivery of safe and effective treatments to patients. To read the full study click here.
